当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Copper‐Catalyzed Enantioselective Construction of Tertiary Propargylic Sulfones
Angewandte Chemie International Edition ( IF 16.6 ) Pub Date : 2019-02-15 , DOI: 10.1002/anie.201814242 José Enrique Gómez 1 , Àlex Cristòfol 1 , Arjan W. Kleij 1, 2
Angewandte Chemie International Edition ( IF 16.6 ) Pub Date : 2019-02-15 , DOI: 10.1002/anie.201814242 José Enrique Gómez 1 , Àlex Cristòfol 1 , Arjan W. Kleij 1, 2
Affiliation
|
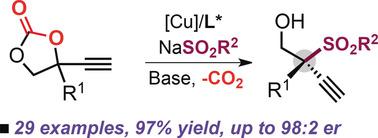
Tertiary propargylic sulfones are of significant importance in organic synthesis and medicinal chemistry, but to date no general asymmetric synthesis approach has been developed. We disclose a versatile copper‐catalyzed sulfonylation of propargylic cyclic carbonates using sodium sulfinates that allows the construction of propargylic sulfones featuring elusive quaternary stereocenters. This method provides the first successful example of such an enantioselective propargylic sulfonylation, features high asymmetric induction, wide functional group tolerance, and scalability, and enables attractive product diversification.
中文翻译:

铜催化叔丙基砜的对映选择性构建
叔炔丙基砜在有机合成和药物化学中具有重要意义,但迄今为止,尚未开发出通用的不对称合成方法。我们公开了使用亚磺酸钠的多官能铜催化的炔丙基环状碳酸酯的磺酰化反应,该方法可以构建具有难以捉摸的四元立体中心的炔丙基砜。该方法提供了这种对映选择性炔丙基磺酰化的第一个成功实例,具有高不对称诱导,宽泛的官能团耐受性和可扩展性,并能实现有吸引力的产品多样化。
更新日期:2019-02-15
中文翻译:

铜催化叔丙基砜的对映选择性构建
叔炔丙基砜在有机合成和药物化学中具有重要意义,但迄今为止,尚未开发出通用的不对称合成方法。我们公开了使用亚磺酸钠的多官能铜催化的炔丙基环状碳酸酯的磺酰化反应,该方法可以构建具有难以捉摸的四元立体中心的炔丙基砜。该方法提供了这种对映选择性炔丙基磺酰化的第一个成功实例,具有高不对称诱导,宽泛的官能团耐受性和可扩展性,并能实现有吸引力的产品多样化。



























 京公网安备 11010802027423号
京公网安备 11010802027423号