当前位置:
X-MOL 学术
›
Genet. Med.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
CYP2D6-guided opioid therapy improves pain control in CYP2D6 intermediate and poor metabolizers: a pragmatic clinical trial.
Genetics in Medicine ( IF 6.6 ) Pub Date : 2019-01-23 , DOI: 10.1038/s41436-018-0431-8 D Max Smith 1 , Kristin W Weitzel 1, 2 , Amanda R Elsey 1, 3 , Taimour Langaee 1, 2 , Yan Gong 1, 2 , Dyson T Wake 1 , Benjamin Q Duong 1 , Melanie Hagen 4 , Christopher A Harle 5 , Elvira Mercado 6 , Ying Nagoshi 4 , Kimberly Newsom 7 , Ashleigh Wright 4 , Eric I Rosenberg 4 , Petr Starostik 7, 8 , Michael J Clare-Salzler 7 , Siegfried O Schmidt 6 , Roger B Fillingim 3, 9 , Julie A Johnson 1, 2, 3 , Larisa H Cavallari 1, 2, 3
Genetics in Medicine ( IF 6.6 ) Pub Date : 2019-01-23 , DOI: 10.1038/s41436-018-0431-8 D Max Smith 1 , Kristin W Weitzel 1, 2 , Amanda R Elsey 1, 3 , Taimour Langaee 1, 2 , Yan Gong 1, 2 , Dyson T Wake 1 , Benjamin Q Duong 1 , Melanie Hagen 4 , Christopher A Harle 5 , Elvira Mercado 6 , Ying Nagoshi 4 , Kimberly Newsom 7 , Ashleigh Wright 4 , Eric I Rosenberg 4 , Petr Starostik 7, 8 , Michael J Clare-Salzler 7 , Siegfried O Schmidt 6 , Roger B Fillingim 3, 9 , Julie A Johnson 1, 2, 3 , Larisa H Cavallari 1, 2, 3
Affiliation
|
PURPOSE
CYP2D6 bioactivates codeine and tramadol, with intermediate and poor metabolizers (IMs and PMs) expected to have impaired analgesia. This pragmatic proof-of-concept trial tested the effects of CYP2D6-guided opioid prescribing on pain control.
METHODS
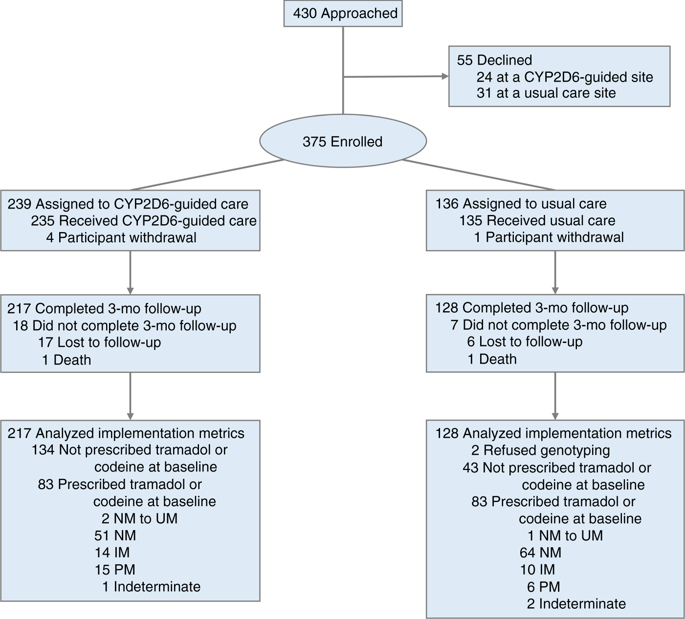
Participants with chronic pain (94% on an opioid) from seven clinics were enrolled into CYP2D6-guided (n = 235) or usual care (n = 135) arms using a cluster design. CYP2D6 phenotypes were assigned based on genotype and CYP2D6 inhibitor use, with recommendations for opioid prescribing made in the CYP2D6-guided arm. Pain was assessed at baseline and 3 months using PROMIS® measures.
RESULTS
On stepwise multiple linear regression, the primary outcome of composite pain intensity (composite of current pain and worst and average pain in the past week) among IM/PMs initially prescribed tramadol/codeine (n = 45) had greater improvement in the CYP2D6-guided versus usual care arm (-1.01 ± 1.59 vs. -0.40 ± 1.20; adj P = 0.016); 24% of CYP2D6-guided versus 0% of usual care participants reported ≥30% (clinically meaningful) reduction in the composite outcome. In contrast, among normal metabolizers prescribed tramadol or codeine at baseline, there was no difference in the change in composite pain intensity at 3 months between CYP2D6-guided (-0.61 ± 1.39) and usual care (-0.54 ± 1.69) groups (adj P = 0.540).
CONCLUSION
These data support the potential benefits of CYP2D6-guided pain management.
中文翻译:

CYP2D6 指导的阿片类药物治疗可改善 CYP2D6 中度和差代谢者的疼痛控制:一项实用的临床试验。
目的 CYP2D6 可生物激活可待因和曲马多,中等和低代谢者(IMs 和 PMs)预计会受损镇痛。这项实用的概念验证试验测试了 CYP2D6 指导的阿片类药物处方对疼痛控制的影响。方法 使用集群设计将来自 7 个诊所的慢性疼痛患者(94% 使用阿片类药物)纳入 CYP2D6 指导(n = 235)或常规护理(n = 135)组。根据基因型和 CYP2D6 抑制剂的使用情况分配 CYP2D6 表型,并在 CYP2D6 指导的手臂中提出阿片类药物处方建议。使用 PROMIS® 措施在基线和 3 个月时评估疼痛。结果 在逐步多元线性回归中,在最初开具曲马多/可待因(n = 45)的 IM/PM 中,复合疼痛强度(当前疼痛与过去一周最严重和平均疼痛的复合)的主要结果在 CYP2D6 指导下与常规护理组相比有更大的改善(- 1.01 ± 1.59 与 -0.40 ± 1.20;调整 P = 0.016);24% 的 CYP2D6 指导和 0% 的常规护理参与者报告复合结果减少 ≥30%(具有临床意义)。相比之下,在基线时开具曲马多或可待因的正常代谢者中,CYP2D6 指导 (-0.61 ± 1.39) 和常规护理 (-0.54 ± 1.69) 组之间 3 个月时复合疼痛强度的变化没有差异 (adj P = 0.540)。结论 这些数据支持 CYP2D6 指导的疼痛管理的潜在益处。59 对 -0.40 ± 1.20;调整 P = 0.016); 24% 的 CYP2D6 指导和 0% 的常规护理参与者报告复合结果减少 ≥30%(具有临床意义)。相比之下,在基线时开具曲马多或可待因的正常代谢者中,CYP2D6 指导 (-0.61 ± 1.39) 和常规护理 (-0.54 ± 1.69) 组之间 3 个月时复合疼痛强度的变化没有差异 (adj P = 0.540)。结论 这些数据支持 CYP2D6 指导的疼痛管理的潜在益处。59 对 -0.40 ± 1.20;调整 P = 0.016); 24% 的 CYP2D6 指导和 0% 的常规护理参与者报告复合结果减少 ≥30%(具有临床意义)。相比之下,在基线时开具曲马多或可待因的正常代谢者中,CYP2D6 指导 (-0.61 ± 1.39) 和常规护理 (-0.54 ± 1.69) 组之间 3 个月时复合疼痛强度的变化没有差异 (adj P = 0.540)。结论 这些数据支持 CYP2D6 指导的疼痛管理的潜在益处。CYP2D6 指导 (-0.61 ± 1.39) 和常规护理 (-0.54 ± 1.69) 组之间 3 个月时复合疼痛强度的变化没有差异 (adj P = 0.540)。结论 这些数据支持 CYP2D6 指导的疼痛管理的潜在益处。CYP2D6 指导 (-0.61 ± 1.39) 和常规护理 (-0.54 ± 1.69) 组之间 3 个月时复合疼痛强度的变化没有差异 (adj P = 0.540)。结论 这些数据支持 CYP2D6 指导的疼痛管理的潜在益处。
更新日期:2019-01-26
中文翻译:

CYP2D6 指导的阿片类药物治疗可改善 CYP2D6 中度和差代谢者的疼痛控制:一项实用的临床试验。
目的 CYP2D6 可生物激活可待因和曲马多,中等和低代谢者(IMs 和 PMs)预计会受损镇痛。这项实用的概念验证试验测试了 CYP2D6 指导的阿片类药物处方对疼痛控制的影响。方法 使用集群设计将来自 7 个诊所的慢性疼痛患者(94% 使用阿片类药物)纳入 CYP2D6 指导(n = 235)或常规护理(n = 135)组。根据基因型和 CYP2D6 抑制剂的使用情况分配 CYP2D6 表型,并在 CYP2D6 指导的手臂中提出阿片类药物处方建议。使用 PROMIS® 措施在基线和 3 个月时评估疼痛。结果 在逐步多元线性回归中,在最初开具曲马多/可待因(n = 45)的 IM/PM 中,复合疼痛强度(当前疼痛与过去一周最严重和平均疼痛的复合)的主要结果在 CYP2D6 指导下与常规护理组相比有更大的改善(- 1.01 ± 1.59 与 -0.40 ± 1.20;调整 P = 0.016);24% 的 CYP2D6 指导和 0% 的常规护理参与者报告复合结果减少 ≥30%(具有临床意义)。相比之下,在基线时开具曲马多或可待因的正常代谢者中,CYP2D6 指导 (-0.61 ± 1.39) 和常规护理 (-0.54 ± 1.69) 组之间 3 个月时复合疼痛强度的变化没有差异 (adj P = 0.540)。结论 这些数据支持 CYP2D6 指导的疼痛管理的潜在益处。59 对 -0.40 ± 1.20;调整 P = 0.016); 24% 的 CYP2D6 指导和 0% 的常规护理参与者报告复合结果减少 ≥30%(具有临床意义)。相比之下,在基线时开具曲马多或可待因的正常代谢者中,CYP2D6 指导 (-0.61 ± 1.39) 和常规护理 (-0.54 ± 1.69) 组之间 3 个月时复合疼痛强度的变化没有差异 (adj P = 0.540)。结论 这些数据支持 CYP2D6 指导的疼痛管理的潜在益处。59 对 -0.40 ± 1.20;调整 P = 0.016); 24% 的 CYP2D6 指导和 0% 的常规护理参与者报告复合结果减少 ≥30%(具有临床意义)。相比之下,在基线时开具曲马多或可待因的正常代谢者中,CYP2D6 指导 (-0.61 ± 1.39) 和常规护理 (-0.54 ± 1.69) 组之间 3 个月时复合疼痛强度的变化没有差异 (adj P = 0.540)。结论 这些数据支持 CYP2D6 指导的疼痛管理的潜在益处。CYP2D6 指导 (-0.61 ± 1.39) 和常规护理 (-0.54 ± 1.69) 组之间 3 个月时复合疼痛强度的变化没有差异 (adj P = 0.540)。结论 这些数据支持 CYP2D6 指导的疼痛管理的潜在益处。CYP2D6 指导 (-0.61 ± 1.39) 和常规护理 (-0.54 ± 1.69) 组之间 3 个月时复合疼痛强度的变化没有差异 (adj P = 0.540)。结论 这些数据支持 CYP2D6 指导的疼痛管理的潜在益处。











































 京公网安备 11010802027423号
京公网安备 11010802027423号