当前位置:
X-MOL 学术
›
Acc. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Bispyrene-Based Self-Assembled Nanomaterials: In Vivo Self-Assembly, Transformation, and Biomedical Effects.
Accounts of Chemical Research ( IF 18.3 ) Pub Date : 2019-01-17 , DOI: 10.1021/acs.accounts.8b00398 Ping-Ping He 1, 2 , Xiang-Dan Li 1 , Lei Wang 2 , Hao Wang 2, 3
Accounts of Chemical Research ( IF 18.3 ) Pub Date : 2019-01-17 , DOI: 10.1021/acs.accounts.8b00398 Ping-Ping He 1, 2 , Xiang-Dan Li 1 , Lei Wang 2 , Hao Wang 2, 3
Affiliation
|
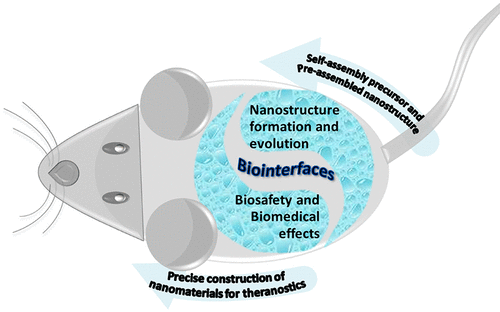
Self-assembled nanomaterials show potential high efficiency as theranostic agents for high-performance imaging and therapy. However, superstructures and properties of preassembled nanomaterials are somewhat compromised under complicated physiological conditions. Given the advantages of the dynamic nature and adaptive behavior of self-assembly systems, we propose an "in vivo self-assembly" strategy for in situ construction of nanomaterials in living objects. For the proof-of-concept study of in vivo self-assembly, we developed a bispyrene (BP) molecule as a multifunctional building block. BP molecules show nonfluorescence in the monomeric state. Quantum-chemical calculations indicate that BP forms twisted intramolecular charge transfer states, which are separated into two orthogonal units, preventing the fluorescence emission. Interestingly, the typical excimeric emission of BP is observed with the formation of J-type aggregates, as confirmed by single-crystal X-ray diffraction. Packing of the BP molecules generates parallel pyrene units that interact with adjacent ones in a slipped face-to-face fashion through intermolecular π-π interactions. BP and/or its amphiphilic derivatives are capable of self-aggregating into nanoparticles (NPs) in aqueous solution because of the hydrophobic and π-π interactions of BP. Upon specific biological stimuli, BP NPs can be transformed into variable self-assembled superstructures. Importantly, the self-assembled BP NPs exhibit turn-on fluorescence signals that can be used to monitor the self-assembly/disassembly process in vitro and in vivo. On the basis of the photophysical properties of BP and its aggregates, we synthesized a series of designed BP derivatives as building blocks for in situ construction of functional nanomaterials for bioimaging and/or therapeutics. We observed several new biomedical effects, e.g., (i) the assembly/aggregation-induced retention (AIR) effect, which shows improved accumulation and retention of bioactive nanomaterials in the regions of interests; (ii) the transformation-induced surface adhesion (TISA) effect, which means the BP NPs transform into nanofibers (NFs) on cell surfaces upon binding with specific receptors, which leads to less uptake of BP NPs by cells via traditional endocytosis pathway; and (iii) transformation of the BP NPs into NFs in the tumor microenvironment, showing high accumulation and long-term retention, revealing the transformation-enhanced accumulation and retention (TEAR) effect. In this Account, we summarize the fluorescence property and emission mechanism of BP building blocks upon aggregation in the biological environment. Moreover, BP-derived compounds used for in vivo self-assembly and transformation are introduced involving modulation strategies. Subsequently, unexpected biomedical effects and applications for theranostics of BP based nanomaterials are discussed. We finally conclude with an outlook toward future developments of BP-based self-assembled nanomaterials.
中文翻译:

基于双sp烯的自组装纳米材料:体内自组装,转化和生物医学效应。
自组装纳米材料作为用于高性能成像和治疗的治疗诊断剂显示出潜在的高效率。然而,在复杂的生理条件下,预组装的纳米材料的上层结构和性能有些受损。鉴于自组装系统的动态性质和自适应行为的优势,我们提出了一种在生物体内原位构建纳米材料的“体内自组装”策略。为了进行体内自组装的概念验证研究,我们开发了双py(BP)分子作为多功能构建基块。BP分子在单体状态下显示非荧光。量子化学计算表明,BP形成扭曲的分子内电荷转移态,将其分成两个正交的单元,从而阻止了荧光发射。有趣的是,通过单晶X射线衍射证实,在形成J型聚集体时观察到了BP的典型激基发射。BP分子的堆积会生成平行的pyr单元,它们通过分子间π-π相互作用以面对面滑动的方式与相邻的pyr单元相互作用。由于BP的疏水和π-π相互作用,BP和/或其两亲衍生物能够在水溶液中自聚集成纳米颗粒(NP)。在特定的生物刺激下,BP NP可以转化为可变的自组装超结构。重要的是,自组装的BP NPs表现出开启的荧光信号,可用于在体外和体内监测自组装/拆卸过程。根据BP及其聚集体的光物理性质,我们合成了一系列设计的BP衍生物作为原位构建用于生物成像和/或治疗的功能纳米材料的基础。我们观察到了几种新的生物医学效应,例如:(i)组装/聚集诱导的保留(AIR)效应,表明感兴趣区域生物活性纳米材料的积累和保留得到改善;(ii)转化诱导的表面粘附(TISA)效应,这意味着BP NP与特定受体结合后会在细胞表面转化为纳米纤维(NFs),从而导致细胞通过传统的内吞途径减少对BP NP的吸收; (iii)在肿瘤微环境中将BP NPs转化为NFs,表现出高积累和长期保留,揭示了转化增强的积累和保留(TEAR)效应。在这个帐户中,我们总结了在生物环境中聚集后BP构建基块的荧光性质和发射机理。此外,引入了用于体内自组装和转化的BP衍生的化合物,其涉及调节策略。随后,讨论了基于BP的纳米材料的意料之外的生物医学作用和应用。最后,我们以对基于BP的自组装纳米材料未来发展的展望作为结束。讨论了基于BP的纳米材料的意料之外的生物医学效应及其在治疗学上的应用。最后,我们以对基于BP的自组装纳米材料未来发展的展望作为结束。讨论了基于BP的纳米材料的意料之外的生物医学效应及其在治疗学上的应用。最后,我们以对基于BP的自组装纳米材料未来发展的展望作为结束。
更新日期:2019-01-17
中文翻译:

基于双sp烯的自组装纳米材料:体内自组装,转化和生物医学效应。
自组装纳米材料作为用于高性能成像和治疗的治疗诊断剂显示出潜在的高效率。然而,在复杂的生理条件下,预组装的纳米材料的上层结构和性能有些受损。鉴于自组装系统的动态性质和自适应行为的优势,我们提出了一种在生物体内原位构建纳米材料的“体内自组装”策略。为了进行体内自组装的概念验证研究,我们开发了双py(BP)分子作为多功能构建基块。BP分子在单体状态下显示非荧光。量子化学计算表明,BP形成扭曲的分子内电荷转移态,将其分成两个正交的单元,从而阻止了荧光发射。有趣的是,通过单晶X射线衍射证实,在形成J型聚集体时观察到了BP的典型激基发射。BP分子的堆积会生成平行的pyr单元,它们通过分子间π-π相互作用以面对面滑动的方式与相邻的pyr单元相互作用。由于BP的疏水和π-π相互作用,BP和/或其两亲衍生物能够在水溶液中自聚集成纳米颗粒(NP)。在特定的生物刺激下,BP NP可以转化为可变的自组装超结构。重要的是,自组装的BP NPs表现出开启的荧光信号,可用于在体外和体内监测自组装/拆卸过程。根据BP及其聚集体的光物理性质,我们合成了一系列设计的BP衍生物作为原位构建用于生物成像和/或治疗的功能纳米材料的基础。我们观察到了几种新的生物医学效应,例如:(i)组装/聚集诱导的保留(AIR)效应,表明感兴趣区域生物活性纳米材料的积累和保留得到改善;(ii)转化诱导的表面粘附(TISA)效应,这意味着BP NP与特定受体结合后会在细胞表面转化为纳米纤维(NFs),从而导致细胞通过传统的内吞途径减少对BP NP的吸收; (iii)在肿瘤微环境中将BP NPs转化为NFs,表现出高积累和长期保留,揭示了转化增强的积累和保留(TEAR)效应。在这个帐户中,我们总结了在生物环境中聚集后BP构建基块的荧光性质和发射机理。此外,引入了用于体内自组装和转化的BP衍生的化合物,其涉及调节策略。随后,讨论了基于BP的纳米材料的意料之外的生物医学作用和应用。最后,我们以对基于BP的自组装纳米材料未来发展的展望作为结束。讨论了基于BP的纳米材料的意料之外的生物医学效应及其在治疗学上的应用。最后,我们以对基于BP的自组装纳米材料未来发展的展望作为结束。讨论了基于BP的纳米材料的意料之外的生物医学效应及其在治疗学上的应用。最后,我们以对基于BP的自组装纳米材料未来发展的展望作为结束。



























 京公网安备 11010802027423号
京公网安备 11010802027423号