当前位置:
X-MOL 学术
›
J. Hydrol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Geochemical evolution of thermal waters in carbonate – evaporitic systems: the triggering effect of halite dissolution in the dedolomitisation and albitisation processes
Journal of Hydrology ( IF 5.9 ) Pub Date : 2019-03-01 , DOI: 10.1016/j.jhydrol.2019.01.013 Mónica Blasco , Luis F. Auqué , María J. Gimeno
Journal of Hydrology ( IF 5.9 ) Pub Date : 2019-03-01 , DOI: 10.1016/j.jhydrol.2019.01.013 Mónica Blasco , Luis F. Auqué , María J. Gimeno
|
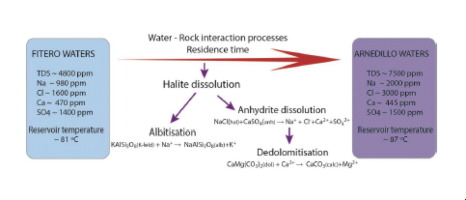
Abstract The Fitero and Arnedillo geothermal systems are located in the NW part of the Iberian Range (Northern Spain). The geothermal reservoir is hosted in the Lower Jurassic carbonates, in contact with the evaporitic Keuper Facies. Thermal waters are of chloride-sodium type with discharge temperature of about 45 °C and near neutral pH. The Arnedillo waters are more saline with higher Na, Cl and sulphate contents, but lower Ca and Mg than the Fitero waters. All waters have attained mineral equilibrium at depth with calcite, dolomite, anhydrite, quartz, albite, K-feldspar and other aluminosilicates, except for the Fitero waters, which have not reached the equilibrium with the aluminosilicates. The calculated reservoir temperature is 81 ± 11 °C in Fitero and 87 ± 13 °C in Arnedillo. In order to identify the reasons for the differences found between the two systems some inverse and forward geochemical calculations were performed and the main water-rock interaction processes responsible for the chemical evolution of these waters have been evaluated. Halite dissolution has been found to be the triggering factor for the two most important geochemical processes in the system: a) albitisation process, due to the common ion effect (Na); and b) dedolomitisation process, associated with the salinity increase, which enhance the dissolution of anhydrite and, in turn, produces the precipitation of calcite (common ion effect, Ca) and the concomitant dissolution of dolomite. Halite dissolution may be an important driving force in the geochemical evolution of groundwater systems in contact with carbonates and evaporites, where equilibrium with K-feldspar, albite and anhydrite has already been attained. The evolution of the processes at pH, temperature and salinity ranges wider than those in the Fitero-Arnedillo system has been theoretically examined with additional reaction-path simulations, in order to generalise the geochemical behaviour of these processes in other environments.
中文翻译:

碳酸盐岩-蒸发系统中热水的地球化学演化:岩盐溶解在脱云石化和石化过程中的触发效应
摘要 Fitero 和 Arnedillo 地热系统位于伊比利亚山脉(西班牙北部)的西北部分。地热储层位于下侏罗统碳酸盐岩中,与蒸发的 Keuper 相接触。温泉水为氯化钠型,排放温度约为 45 °C,pH 值接近中性。Arnedillo 水域含盐量更高,Na、Cl 和硫酸盐含量更高,但 Ca 和 Mg 含量低于 Fitero 水域。除Fitero 水域未与铝硅酸盐达到平衡外,所有水域均已与方解石、白云石、硬石膏、石英、钠长石、钾长石和其他铝硅酸盐在深度处达到矿物平衡。计算出的水库温度在 Fitero 为 81 ± 11 °C,在 Arnedillo 为 87 ± 13 °C。为了确定两个系统之间发现差异的原因,我们进行了一些逆向和正向地球化学计算,并对导致这些水化学演化的主要水岩相互作用过程进行了评估。已发现岩盐溶解是该系统中两个最重要的地球化学过程的触发因素: a) 由于共同离子效应 (Na) 引起的白化过程;b) 脱云石化过程,伴随着盐度的增加,这增强了硬石膏的溶解,进而产生了方解石的沉淀(共离子效应,Ca)和白云石的伴随溶解。岩盐溶解可能是与碳酸盐和蒸发岩接触的地下水系统地球化学演化的重要驱动力,其中钾长石、钠长石和硬石膏已经达到平衡。在 pH、温度和盐度范围比 Fitero-Arnedillo 系统更宽的过程的演变已经通过额外的反应路径模拟进行了理论上的检查,以便概括这些过程在其他环境中的地球化学行为。
更新日期:2019-03-01
中文翻译:

碳酸盐岩-蒸发系统中热水的地球化学演化:岩盐溶解在脱云石化和石化过程中的触发效应
摘要 Fitero 和 Arnedillo 地热系统位于伊比利亚山脉(西班牙北部)的西北部分。地热储层位于下侏罗统碳酸盐岩中,与蒸发的 Keuper 相接触。温泉水为氯化钠型,排放温度约为 45 °C,pH 值接近中性。Arnedillo 水域含盐量更高,Na、Cl 和硫酸盐含量更高,但 Ca 和 Mg 含量低于 Fitero 水域。除Fitero 水域未与铝硅酸盐达到平衡外,所有水域均已与方解石、白云石、硬石膏、石英、钠长石、钾长石和其他铝硅酸盐在深度处达到矿物平衡。计算出的水库温度在 Fitero 为 81 ± 11 °C,在 Arnedillo 为 87 ± 13 °C。为了确定两个系统之间发现差异的原因,我们进行了一些逆向和正向地球化学计算,并对导致这些水化学演化的主要水岩相互作用过程进行了评估。已发现岩盐溶解是该系统中两个最重要的地球化学过程的触发因素: a) 由于共同离子效应 (Na) 引起的白化过程;b) 脱云石化过程,伴随着盐度的增加,这增强了硬石膏的溶解,进而产生了方解石的沉淀(共离子效应,Ca)和白云石的伴随溶解。岩盐溶解可能是与碳酸盐和蒸发岩接触的地下水系统地球化学演化的重要驱动力,其中钾长石、钠长石和硬石膏已经达到平衡。在 pH、温度和盐度范围比 Fitero-Arnedillo 系统更宽的过程的演变已经通过额外的反应路径模拟进行了理论上的检查,以便概括这些过程在其他环境中的地球化学行为。











































 京公网安备 11010802027423号
京公网安备 11010802027423号