PLOS ONE ( IF 3.7 ) Pub Date : 2019-01-16 , DOI: 10.1371/journal.pone.0210785 Gareth O. Griffiths , Richard A. Cowan , Kenneth M. Grigor , Barbara M. Uscinska , Matthew Sydes , Martin Russell
|
Background
Pure squamous cell carcinoma (SCC) of the urinary tract is rare in the UK and has a poor prognosis compared with transitional cell carcinoma (TCC). Cisplatin based chemotherapy has been shown to be effective in TCC.
Methods
Patients with T3-T4, pelvic relapsed, nodal or metastatic SCC of the urinary tract were recruited into an open-label, single-arm, non-randomised, phase 2 trial evaluating the activity and safety of cisplatin, methotrexate and vinblastine (CMV) chemotherapy. CMV was given as three 21-day cycles of methotrexate 30mg/m2 (day 1 & 8), vinblastine 4mg/m2 (day 1 & 8) and cisplatin 100mg/m2 (day 2).
Results
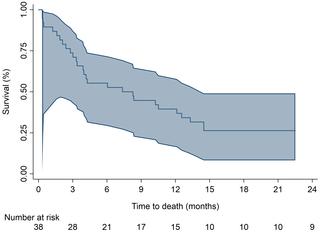
38 patients were recruited. Overall response was 39% (95% CI 24%, 55%)–13% CR and 26% PR. Median OS was 7.8 months (95% CI 3.4, 12.6) with 39% 1-year survival. Toxicity was acceptable.
Conclusion
CMV is well tolerated and active in patients with pure SCC of the urinary tract.
中文翻译:

BA08:顺铂,甲氨蝶呤和长春碱(CMV)治疗泌尿道纯鳞癌的开放标签,单臂,非随机,2期临床试验
背景
纯尿道鳞状细胞癌(SCC)在英国很少见,与移行细胞癌(TCC)相比,预后较差。已显示基于顺铂的化学疗法在TCC中有效。
方法
招募患有T3-T4,骨盆复发,淋巴结转移或转移性SCC的患者参加一项开放标签,单组,非随机,2期试验,评估顺铂,甲氨蝶呤和长春碱(CMV)的活性和安全性化学疗法。给予CMV的三个21天周期为甲氨蝶呤30mg / m 2(第1和8天),长春碱4mg / m 2(第1和8天)和顺铂100mg / m 2(第2天)。
结果
招募了38名患者。总体反应为39%(95%CI 24%,55%)– 13%CR和26%PR。OS中位数为7.8个月(95%CI 3.4,12.6),一年生存率为39%。毒性是可以接受的。
结论
单纯尿路SCC患者对CMV的耐受性良好且活跃。



























 京公网安备 11010802027423号
京公网安备 11010802027423号