当前位置:
X-MOL 学术
›
ACS Earth Space Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Role of Trace Elements in the 226-Radium Incorporation in Sulfate Minerals (Gypsum and Celestite)
ACS Earth and Space Chemistry ( IF 3.4 ) Pub Date : 2019-01-16 00:00:00 , DOI: 10.1021/acsearthspacechem.8b00150 Leslie Lestini 1, 2, 3 , Catherine Beaucaire 1, 2 , Thomas Vercouter 2, 4 , Marine Ballini 3 , Michaël Descostes 3
ACS Earth and Space Chemistry ( IF 3.4 ) Pub Date : 2019-01-16 00:00:00 , DOI: 10.1021/acsearthspacechem.8b00150 Leslie Lestini 1, 2, 3 , Catherine Beaucaire 1, 2 , Thomas Vercouter 2, 4 , Marine Ballini 3 , Michaël Descostes 3
Affiliation
|
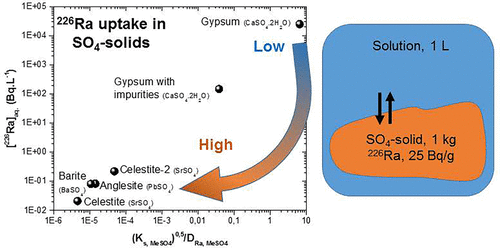
Incorporation of 226Ra within gypsum (CaSO4·2H2O(s)) and celestite (SrSO4(s)) was assessed through dedicated batch experiments monitored over hundreds of days. Dissolution/recrystallization and coprecipitation experiments were carried out to investigate a range of chemical conditions, close to or far from equilibrium conditions. These data were used to establish the ability of gypsum and celestite to incorporate 226Ra. Celestite shows a high 226Ra incorporation, with a partition coefficient around 105. On the contrary, 226Ra is not significantly incorporated into pure gypsum. A very low value of (8.8 ± 1.5) × 10–4 has been estimated, which is much lower than the values reported in the literature. This may explain why 226Ra incorporation in gypsum is enhanced when Sr impurities are present (above 0.1 molar %). Under such conditions, the radium distribution coefficient is around 0.15 ± 0.09. This behavior can be explained by an ion-exchange mechanism between 226Ra and Sr, consistently with the existence of a solid solution between celestite and radium sulfate, similarly to the barite (BaSO4(s)) and radium sulfate solid solution. These results highlight the key role of trace compounds in the incorporation of 226Ra in sulfate bearing minerals and bring new insights in our understanding of the 226Ra behavior in the environment. This is illustrated by the radium content observed and modeled within the pore waters of uranium mining tailings.
中文翻译:

微量元素在硫酸盐矿物质(石膏和天青石)中226-放射性结合中的作用
通过在数百天内监控的专用批处理实验评估了226 Ra在石膏(CaSO 4 ·2H 2 O (s))和天青石(SrSO 4(s))中的掺入。进行了溶解/重结晶和共沉淀实验,以研究接近或远离平衡条件的一系列化学条件。这些数据用于确定石膏和天青石掺入226 Ra的能力。天青石显示出较高的226 Ra掺入,分配系数约为105。相反,226 Ra没有明显掺入纯石膏中。(8.8±1.5)×10 –4的极低值据估计,它远低于文献报道的值。这可以解释为什么当存在Sr杂质(大于0.1摩尔%)时,增强226 Ra掺入石膏的原因。在这种条件下,镭的分布系数约为0.15±0.09。这种行为可以通过226 Ra和Sr之间的离子交换机理来解释,这与天青石和硫酸镭之间存在固溶体相一致,类似于重晶石(BaSO 4(s))和硫酸镭固溶体。这些结果突出了微量化合物在含硫酸盐矿物中掺入226 Ra的关键作用,并为我们对226的理解提供了新的见解。Ra在环境中的行为。铀开采尾矿孔隙水中观察到的和模拟出的镭含量可以说明这一点。
更新日期:2019-01-16
中文翻译:

微量元素在硫酸盐矿物质(石膏和天青石)中226-放射性结合中的作用
通过在数百天内监控的专用批处理实验评估了226 Ra在石膏(CaSO 4 ·2H 2 O (s))和天青石(SrSO 4(s))中的掺入。进行了溶解/重结晶和共沉淀实验,以研究接近或远离平衡条件的一系列化学条件。这些数据用于确定石膏和天青石掺入226 Ra的能力。天青石显示出较高的226 Ra掺入,分配系数约为105。相反,226 Ra没有明显掺入纯石膏中。(8.8±1.5)×10 –4的极低值据估计,它远低于文献报道的值。这可以解释为什么当存在Sr杂质(大于0.1摩尔%)时,增强226 Ra掺入石膏的原因。在这种条件下,镭的分布系数约为0.15±0.09。这种行为可以通过226 Ra和Sr之间的离子交换机理来解释,这与天青石和硫酸镭之间存在固溶体相一致,类似于重晶石(BaSO 4(s))和硫酸镭固溶体。这些结果突出了微量化合物在含硫酸盐矿物中掺入226 Ra的关键作用,并为我们对226的理解提供了新的见解。Ra在环境中的行为。铀开采尾矿孔隙水中观察到的和模拟出的镭含量可以说明这一点。



























 京公网安备 11010802027423号
京公网安备 11010802027423号