Microporous and Mesoporous Materials ( IF 5.2 ) Pub Date : 2019-01-16 , DOI: 10.1016/j.micromeso.2019.01.029 Jaewoo Park , Nour F. Attia , Minji Jung , Kiyoung Lee , Hyunchul Oh
|
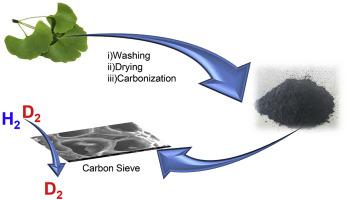
Nuclear fusion as a clean energy source is an approach proposed to solve the expected global energy crisis. Deuterium an isotope of hydrogen, is required as fuel but its conventional separation process is highly energy-intensive. However, selective separation of D2 from H2 gas mixtures using nanoporous materials is a promising solution. To this end, cost-effective nanoporous carbon from agricultural waste has been developed (peanut shell, ginkgo leaf, and metasequoia leaf). Porosity, morphology, and compositional properties of the developed carbon were investigated and compared with commercial activated carbon. The developed carbon achieved a specific surface area of 692 m2/g and a specific pore volume of 0.387 cm3/g with narrow pore size distribution. Hydrogen and deuterium adsorption isotherms were studied at various temperatures (25 K, 40 K, 60 K, and 77 K) for all carbons. D2/H2 selectivity using the ideal adsorption solution theory of equimolar composition and pressures of D2 and H2 was carried out. The D2/H2 selectivity in ginkgo leaf-derived carbon achieved a selectivity value of 4.1 at 25 K, higher than other biomass-derived carbons and commercial activated carbon. Additionally, the heat of adsorption of D2 and H2 for all carbons was evaluated. A large difference between these values was observed in ginkgo leaf-derived carbon. This difference is three times larger than commercial activated carbon, leading to a higher rate of interaction between D2 and carbon than that between H2 and carbon due to rich Ca content in carbon. Thus, metal residues in biomass carbon seem to play a significant role in selective separation of D2 over H2.
中文翻译:

生物基衍生的纳米多孔碳用于氢同位素分离
核聚变作为一种清洁能源是解决预期的全球能源危机的一种方法。氘是氢的同位素,需要用作燃料,但其常规分离过程需要大量能源。然而,使用纳米孔材料从H 2气体混合物中选择性分离D 2是一种有前途的解决方案。为此,已经开发了来自农业废弃物(花生壳,银杏叶和水杉叶)的具有成本效益的纳米多孔碳。研究了已开发碳的孔隙率,形态和组成特性,并将其与市售活性炭进行了比较。所开发的碳的比表面积为692 m 2 / g,比孔容为0.387 cm 3/ g,孔径分布窄。在所有温度下(25 K,40 K,60 K和77 K)研究了氢和氘的吸附等温线。使用等摩尔组成的理想吸附溶液理论和D 2和H 2的压力进行D 2 / H 2选择性。银杏叶衍生碳的D 2 / H 2选择性在25 K时达到4.1的选择性值,高于其他生物质衍生碳和商业活性炭。另外,D 2和H 2的吸附热对所有碳进行了评估。在银杏叶片来源的碳中观察到这些值之间存在很大差异。该差异是商业活性炭的三倍,由于碳中的Ca含量高,导致D 2与碳之间的相互作用速率比H 2与碳之间的相互作用速率更高。因此,生物质碳中的金属残留物似乎在D 2相对于H 2的选择性分离中起着重要作用。



























 京公网安备 11010802027423号
京公网安备 11010802027423号