PLOS ONE ( IF 2.9 ) Pub Date : 2019-01-15 , DOI: 10.1371/journal.pone.0210963 Achim Löf , Gesa König , Sonja Schneppenheim , Reinhard Schneppenheim , Martin Benoit , Ulrich Budde , Jochen P. Müller , Maria A. Brehm
|
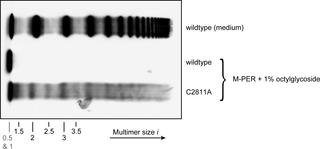
The formation of hemostatic plugs at sites of vascular injury crucially involves the multimeric glycoprotein von Willebrand factor (VWF). VWF multimers are linear chains of N-terminally linked dimers. The latter are formed from monomers via formation of the C-terminal disulfide bonds Cys2771-Cys2773’, Cys2773-Cys2771’, and Cys2811-Cys2811’. Mutations in VWF that impair multimerization can lead to subtype 2A of the bleeding disorder von Willebrand Disease (VWD). Commonly, the multimer size distribution of VWF is assessed by electrophoretic multimer analysis. Here, we present atomic force microscopy (AFM) imaging as a method to determine the size distribution of VWF variants by direct visualization at the single-molecule level. We first validated our approach by investigating recombinant wildtype VWF and a previously studied mutant (p.Cys1099Tyr) that impairs N-terminal multimerization. We obtained excellent quantitative agreement with results from earlier studies and with electrophoretic multimer analysis. We then imaged specific mutants that are known to exhibit disturbed C-terminal dimerization. For the mutants p.Cys2771Arg and p.Cys2773Arg, we found the majority of monomers (87 ± 5% and 73 ± 4%, respectively) not to be C-terminally dimerized. While these results confirm that Cys2771 and Cys2773 are crucial for dimerization, they additionally provide quantitative information on the mutants’ different abilities to form alternative C-terminal disulfides for residual dimerization. We further mutated Cys2811 to Ala and found that only 23 ± 3% of monomers are not C-terminally dimerized, indicating that Cys2811 is structurally less important for dimerization. Furthermore, for mutants p.Cys2771Arg, p.Cys2773Arg, and p.Cys2811Ala we found ‘even-numbered’ non-native multimers, i.e. multimers with monomers attached on both termini; a multimer species that cannot be distinguished from native multimers by conventional multimer analysis. Summarizing, we demonstrate that AFM imaging can provide unique insights into VWF processing defects at the single-molecule level that cannot be gained from established methods of multimer analysis.
中文翻译:

通过单分子原子力显微镜成像推进冯·威勒布兰德因子的多聚体分析
血管损伤部位的止血栓的形成关键涉及多聚糖蛋白von Willebrand因子(VWF)。VWF多聚体是N末端连接的二聚体的线性链。后者通过形成C端二硫键Cys2771-Cys2773',Cys2773-Cys2771'和Cys2811-Cys2811'由单体形成。VWF中影响多聚体的突变可导致出血性疾病von Willebrand病(VWD)的2A亚型。通常,VWF的多聚体尺寸分布是通过电泳多聚体分析来评估的。在这里,我们介绍原子力显微镜(AFM)成像作为一种方法,通过在单分子水平上直接可视化来确定VWF变体的大小分布。我们首先通过研究重组野生型VWF和先前研究的突变体(p。Cys1099Tyr)会损害N端多聚体。我们与早期研究的结果以及电泳多聚体分析获得了极好的定量一致性。然后,我们对已知表现出受干扰的C端二聚化的特定突变体进行了成像。对于突变体p.Cys2771Arg和p.Cys2773Arg,我们发现大多数单体(分别为87±5%和73±4%)没有C端二聚。尽管这些结果证实了Cys2771和Cys2773对于二聚化至关重要,但它们还提供了有关突变体形成剩余C-末端二硫化物以进行残留二聚化的不同能力的定量信息。我们进一步将Cys2811突变为Ala,发现只有23±3%的单体没有C端二聚化,表明Cys2811在结构上对二聚化的重要性较低。此外,对于突变体p.Cys2771Arg,p.Cys2773Arg和p.Cys2811Ala,我们发现了“偶数”非天然多聚体,即两个末端均带有单体的多聚体。通过常规多聚体分析无法将其与天然多聚体区分开的多聚体物种。总而言之,我们证明了AFM成像可以为单分子水平的VWF处理缺陷提供独特的见识,而这些缺陷是无法从已建立的多聚体分析方法中获得的。











































 京公网安备 11010802027423号
京公网安备 11010802027423号