当前位置:
X-MOL 学术
›
ACS Cent. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Principles of Inter-Amino-Acid Recognition Revealed by Binding Energies between Homogeneous Oligopeptides
ACS Central Science ( IF 12.7 ) Pub Date : 2019-01-14 00:00:00 , DOI: 10.1021/acscentsci.8b00723 Huiwen Du 1, 2 , Xiaoyu Hu 3 , Hongyang Duan 1, 2 , Lanlan Yu 1, 2 , Fuyang Qu 1, 2 , Qunxing Huang 1, 2 , Wangshu Zheng 1, 2 , Hanyi Xie 1, 2 , Jiaxi Peng 1, 2 , Rui Tuo 4 , Dan Yu 4 , Yuchen Lin 1, 2 , Wenzhe Li 1, 2 , Yongfang Zheng 1, 2 , Xiaocui Fang 1, 2 , Yimin Zou 1, 2 , Huayi Wang 1, 2 , Mengting Wang 1, 2 , Paul S Weiss 5 , Yanlian Yang 1, 2 , Chen Wang 1, 2, 6
ACS Central Science ( IF 12.7 ) Pub Date : 2019-01-14 00:00:00 , DOI: 10.1021/acscentsci.8b00723 Huiwen Du 1, 2 , Xiaoyu Hu 3 , Hongyang Duan 1, 2 , Lanlan Yu 1, 2 , Fuyang Qu 1, 2 , Qunxing Huang 1, 2 , Wangshu Zheng 1, 2 , Hanyi Xie 1, 2 , Jiaxi Peng 1, 2 , Rui Tuo 4 , Dan Yu 4 , Yuchen Lin 1, 2 , Wenzhe Li 1, 2 , Yongfang Zheng 1, 2 , Xiaocui Fang 1, 2 , Yimin Zou 1, 2 , Huayi Wang 1, 2 , Mengting Wang 1, 2 , Paul S Weiss 5 , Yanlian Yang 1, 2 , Chen Wang 1, 2, 6
Affiliation
|
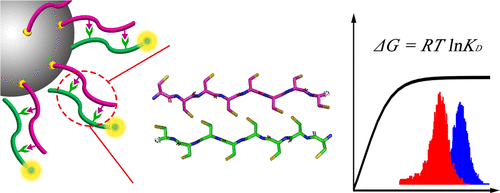
We have determined the interaction strengths of the common naturally occurring amino acids using a complete binding affinity matrix of 20 × 20 pairs of homo-octapeptides consisting of the 20 common amino acids between stationary and mobile states. We used a bead-based fluorescence assay for these measurements. The results provide a basis for analyzing specificity, polymorphisms, and selectivity of inter-amino-acid interactions. Comparative analyses of the binding energies, i.e., the free energies of association (ΔGA), reveal contributions assignable to both main-chain-related and side-chain-related interactions originating from the chemical structures of these 20 common amino acids. Side-chain–side-chain and side-chain–main-chain interactions are found to be pronounced in an identified set of amino acid pairs that determine the basis of inter-amino-acid recognition.
中文翻译:

由同源寡肽之间的结合能揭示的氨基酸间识别原理
我们使用 20 × 20 对同源八肽的完整结合亲和力矩阵确定了常见天然氨基酸的相互作用强度,该矩阵由固定和移动状态之间的 20 种常见氨基酸组成。我们使用基于珠子的荧光测定法进行这些测量。结果为分析氨基酸间相互作用的特异性、多态性和选择性提供了基础。结合能(即缔合自由能 (Δ G A ))的比较分析揭示了源自这 20 种常见氨基酸化学结构的主链相关和侧链相关相互作用的贡献。发现侧链-侧链和侧链-主链相互作用在一组确定的氨基酸对中很明显,这些氨基酸对决定了氨基酸间识别的基础。
更新日期:2019-01-14
中文翻译:

由同源寡肽之间的结合能揭示的氨基酸间识别原理
我们使用 20 × 20 对同源八肽的完整结合亲和力矩阵确定了常见天然氨基酸的相互作用强度,该矩阵由固定和移动状态之间的 20 种常见氨基酸组成。我们使用基于珠子的荧光测定法进行这些测量。结果为分析氨基酸间相互作用的特异性、多态性和选择性提供了基础。结合能(即缔合自由能 (Δ G A ))的比较分析揭示了源自这 20 种常见氨基酸化学结构的主链相关和侧链相关相互作用的贡献。发现侧链-侧链和侧链-主链相互作用在一组确定的氨基酸对中很明显,这些氨基酸对决定了氨基酸间识别的基础。










































 京公网安备 11010802027423号
京公网安备 11010802027423号