当前位置:
X-MOL 学术
›
ACS Cent. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Nanoscale Ion Emitters in Native Mass Spectrometry for Measuring Ligand–Protein Binding Affinities
ACS Central Science ( IF 12.7 ) Pub Date : 2019-01-14 00:00:00 , DOI: 10.1021/acscentsci.8b00787 Giang T. H. Nguyen 1 , Thinh N. Tran 2 , Matthew N. Podgorski 3 , Stephen G. Bell 3 , Claudiu T. Supuran 4 , William A. Donald 1
ACS Central Science ( IF 12.7 ) Pub Date : 2019-01-14 00:00:00 , DOI: 10.1021/acscentsci.8b00787 Giang T. H. Nguyen 1 , Thinh N. Tran 2 , Matthew N. Podgorski 3 , Stephen G. Bell 3 , Claudiu T. Supuran 4 , William A. Donald 1
Affiliation
|
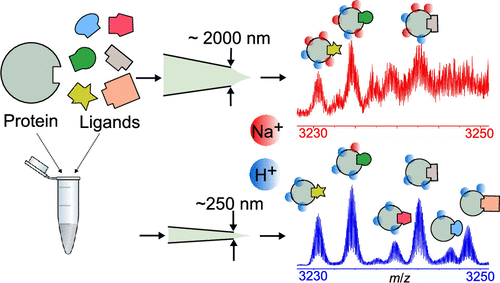
Electrospray ionization (ESI) mass spectrometry (MS) is a crucial method for rapidly determining the interactions between small molecules and proteins with ultrahigh sensitivity. However, nonvolatile molecules and salts that are often necessary to stabilize the native structures of protein–ligand complexes can readily adduct to protein ions, broaden spectral peaks, and lower signal-to-noise ratios in native MS. ESI emitters with narrow tip diameters (∼250 nm) were used to significantly reduce the extent of adduction of salt and nonvolatile molecules to protein complexes to more accurately measure ligand–protein binding constants than by use of conventional larger-bore emitters under these conditions. As a result of decreased salt adduction, peaks corresponding to protein–ligand complexes that differ in relative molecular weight by as low as 0.06% can be readily resolved. For low-molecular-weight anion ligands formed from sodium salts, anion-bound and unbound protein ions that differ in relative mass by 0.2% were completely baseline resolved using nanoscale emitters, which was not possible under these conditions using conventional emitters. Owing to the improved spectral resolution obtained using narrow-bore emitters and an analytically derived equation, Kd values were simultaneously obtained for at least six ligands to a single druggable protein target from one spectrum for the first time. This research suggests that ligand–protein binding constants can be directly and accurately measured from solutions with high concentrations of nonvolatile buffers and salts by native MS.
中文翻译:

天然质谱中的纳米级离子发射体用于测量配体-蛋白质结合亲和力
电喷雾电离(ESI)质谱(MS)是一种以超高灵敏度快速确定小分子与蛋白质之间相互作用的关键方法。然而,稳定蛋白质-配体复合物的天然结构通常必需的非挥发性分子和盐类可以很容易地加成到蛋白质离子上,加宽光谱峰,并降低天然MS中的信噪比。与在这些条件下使用传统的大口径发射器相比,具有窄尖端直径(约250 nm)的ESI发射器可显着减少盐和非挥发性分子与蛋白质复合物的内聚程度,从而更准确地测量配体与蛋白质的结合常数。由于减少了盐的内含,相应于蛋白质-配体复合物的峰在相对分子量上的差异低至0。06%可以轻易解决。对于由钠盐形成的低分子量阴离子配体,使用纳米级发射体将相对质量相差0.2%的阴离子结合和未结合的蛋白质离子完全基线分离,这在常规条件下是不可能的。由于使用窄口径发射器和解析得出的方程式可以提高光谱分辨率,首次从一个光谱中同时获得了至少6个配体与单个可药物化蛋白靶标的K d值。这项研究表明,可以通过天然MS从具有高浓度非挥发性缓冲液和盐的溶液中直接,准确地测量配体与蛋白质的结合常数。
更新日期:2019-01-14
中文翻译:

天然质谱中的纳米级离子发射体用于测量配体-蛋白质结合亲和力
电喷雾电离(ESI)质谱(MS)是一种以超高灵敏度快速确定小分子与蛋白质之间相互作用的关键方法。然而,稳定蛋白质-配体复合物的天然结构通常必需的非挥发性分子和盐类可以很容易地加成到蛋白质离子上,加宽光谱峰,并降低天然MS中的信噪比。与在这些条件下使用传统的大口径发射器相比,具有窄尖端直径(约250 nm)的ESI发射器可显着减少盐和非挥发性分子与蛋白质复合物的内聚程度,从而更准确地测量配体与蛋白质的结合常数。由于减少了盐的内含,相应于蛋白质-配体复合物的峰在相对分子量上的差异低至0。06%可以轻易解决。对于由钠盐形成的低分子量阴离子配体,使用纳米级发射体将相对质量相差0.2%的阴离子结合和未结合的蛋白质离子完全基线分离,这在常规条件下是不可能的。由于使用窄口径发射器和解析得出的方程式可以提高光谱分辨率,首次从一个光谱中同时获得了至少6个配体与单个可药物化蛋白靶标的K d值。这项研究表明,可以通过天然MS从具有高浓度非挥发性缓冲液和盐的溶液中直接,准确地测量配体与蛋白质的结合常数。







































 京公网安备 11010802027423号
京公网安备 11010802027423号