npj Biofilms and Microbiomes ( IF 7.8 ) Pub Date : 2019-01-15 , DOI: 10.1038/s41522-018-0075-0 Ashutosh Kumar 1, 2 , Anwar Alam 1, 3 , Sonam Grover 1 , Saurabh Pandey 4, 5 , Deeksha Tripathi 3, 6 , Monika Kumari 7 , Mamta Rani 8 , Aditi Singh 9 , Yusuf Akhter 10 , Nasreen Z Ehtesham 4 , Seyed E Hasnain 1, 3, 11
|
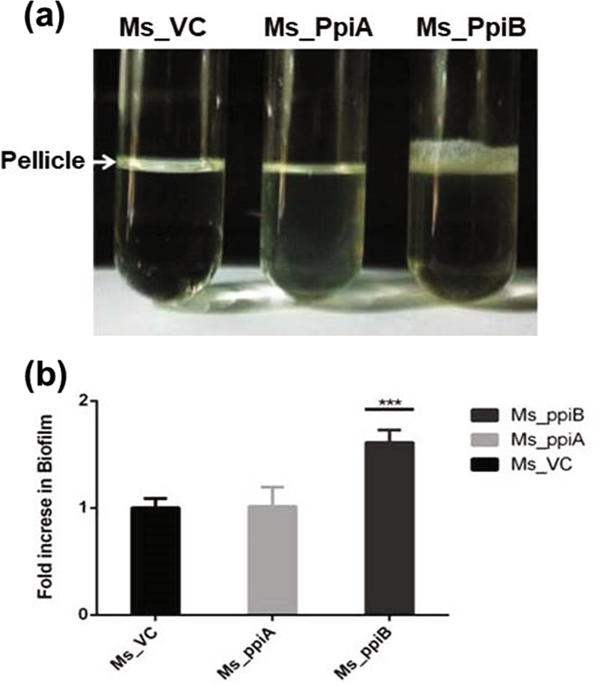
Tuberculosis (TB), a disease caused by Mycobacterium tuberculosis (M.tb), takes one human life every 15 s globally. Disease relapse occurs due to incomplete clearance of the pathogen and reactivation of the antibiotic tolerant bacilli. M.tb, like other bacterial pathogens, creates an ecosystem of biofilm formed by several proteins including the cyclophilins. We show that the M.tb cyclophilin peptidyl-prolyl isomerase (PpiB), an essential gene, is involved in biofilm formation and tolerance to anti-mycobacterial drugs. We predicted interaction between PpiB and US FDA approved drugs (cyclosporine-A and acarbose) by in-silico docking studies and this was confirmed by surface plasmon resonance (SPR) spectroscopy. While all these drugs inhibited growth of Mycobacterium smegmatis (M.smegmatis) when cultured in vitro, acarbose and cyclosporine-A showed bacteriostatic effect while gallium nanoparticle (GaNP) exhibited bactericidal effect. Cyclosporine-A and GaNP additionally disrupted M.tb H37Rv biofilm formation. Co-culturing M.tb in their presence resulted in significant (2–4 fold) decrease in dosage of anti-tubercular drugs- isoniazid and ethambutol. Comparison of the cyclosporine-A and acarbose binding sites in PpiB homologues of other biofilm forming infectious pathogens revealed that these have largely remained unaltered across bacterial species. Targeting bacterial biofilms could be a generic strategy for intervention against bacterial pathogens.
中文翻译:

肽基脯氨酰异构酶-B参与结核分枝杆菌生物膜的形成,并且是基于药物再利用的干预措施的通用靶标。
结核病(TB)是一种由结核分枝杆菌(M.tb)引起的疾病,全球每15 s会使人丧命。由于病原体清除不完全和抗生素耐受性细菌重新活化,导致疾病复发。结核菌与其他细菌病原体一样,会创建由包括亲环蛋白在内的几种蛋白质形成的生物膜生态系统。我们表明,结核分枝杆菌亲环蛋白肽基脯氨酰异构酶(PpiB)是必不可少的基因,它参与生物膜的形成和对抗分枝杆菌药物的耐受性。我们通过计算机对接研究预测了PpiB与美国FDA批准的药物(环孢素A和阿卡波糖)之间的相互作用,这已通过表面等离振子共振(SPR)光谱证实。尽管所有这些药物在体外培养时均能抑制耻垢分枝杆菌(M.smegmatis)的生长,但阿卡波糖和环孢菌素A均具有抑菌作用,而镓纳米颗粒(GaNP)则具有杀菌作用。环孢菌素A和GaNP还会破坏M.tb H 37 Rv生物膜的形成。共培养结核分枝杆菌它们的存在导致抗结核药物异烟肼和乙胺丁醇的剂量显着减少(2-4倍)。比较其他形成生物膜的感染性病原体的PpiB同源物中环孢菌素A和阿卡波糖结合位点,发现这些在细菌种类中基本保持不变。靶向细菌生物膜可能是针对细菌病原体进行干预的通用策略。











































 京公网安备 11010802027423号
京公网安备 11010802027423号