当前位置:
X-MOL 学术
›
Tetrahedron Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
First stereoselective total synthesis of antibiotic macrolide Berkeleylactone F
Tetrahedron Letters ( IF 1.5 ) Pub Date : 2019-01-11 , DOI: 10.1016/j.tetlet.2019.01.017 Mopuri Sudhakar Reddy , Gembali Manikanta , Palakodety Radha Krishna
中文翻译:

首个立体选择性全合成抗生素大环内酯伯克利内酯F
更新日期:2019-01-11
Tetrahedron Letters ( IF 1.5 ) Pub Date : 2019-01-11 , DOI: 10.1016/j.tetlet.2019.01.017 Mopuri Sudhakar Reddy , Gembali Manikanta , Palakodety Radha Krishna
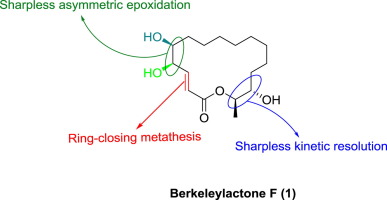
|
The first stereoselective total synthesis of antibiotic macrolide Berkeleylactone F is described. The synthetic sequence notably features Sharpless kinetic resolution to access chiral epoxide followed by its regioselective ring-opening reaction, Sharpless asymmetric reaction and ring-closing metathesis.
中文翻译:

首个立体选择性全合成抗生素大环内酯伯克利内酯F
描述了抗生素大环内酯伯克利内酯F的第一个立体选择性全合成。合成序列的显着特征是通过Sharpless动力学拆分获得手性环氧化物,然后进行区域选择性开环反应,Sharpless不对称反应和闭环复分解反应。











































 京公网安备 11010802027423号
京公网安备 11010802027423号