当前位置:
X-MOL 学术
›
Tetrahedron Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
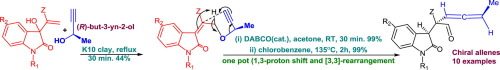
Synthesis of chiral allene moiety from Morita–Baylis–Hillman adduct of isatin derivatives via Claisen rearrangement
Tetrahedron Letters ( IF 1.8 ) Pub Date : 2019-01-11 , DOI: 10.1016/j.tetlet.2019.01.018 Vadivel Vaithiyanathan , Ganesan Ravichandran , Vijayakumar Thirumailavan
中文翻译:

通过Claisen重排从Morita–Baylis–Hillman加成素衍生物的手性异戊烯部分的合成
更新日期:2019-01-11
Tetrahedron Letters ( IF 1.8 ) Pub Date : 2019-01-11 , DOI: 10.1016/j.tetlet.2019.01.018 Vadivel Vaithiyanathan , Ganesan Ravichandran , Vijayakumar Thirumailavan
|
Chiral allenes are synthesized from Morita–Baylis–Hillman adduct of isatin derivatives via Claisen rearrangement for the first time. The chiral 1,3-substituted allene entity is achieved using (R)-but-3-yn-2-ol. The details of the work are elaborately discussed in this letter.
中文翻译:

通过Claisen重排从Morita–Baylis–Hillman加成素衍生物的手性异戊烯部分的合成
手性艾伦是首次通过克莱森重排从森田衍生品的森田-贝利斯-希尔曼加合物合成的。使用(R)-丁-3-yn-2-ol获得手性的1,3-取代的烯丙基实体。这封信详细讨论了工作细节。



























 京公网安备 11010802027423号
京公网安备 11010802027423号