当前位置:
X-MOL 学术
›
ACS Cent. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Effective Distance for DNA-Mediated Charge Transport between Repair Proteins
ACS Central Science ( IF 12.7 ) Pub Date : 2019-01-11 00:00:00 , DOI: 10.1021/acscentsci.8b00566 Edmund C. M. Tse 1 , Theodore J. Zwang 1 , Sebastian Bedoya 1 , Jacqueline K. Barton 1
ACS Central Science ( IF 12.7 ) Pub Date : 2019-01-11 00:00:00 , DOI: 10.1021/acscentsci.8b00566 Edmund C. M. Tse 1 , Theodore J. Zwang 1 , Sebastian Bedoya 1 , Jacqueline K. Barton 1
Affiliation
|
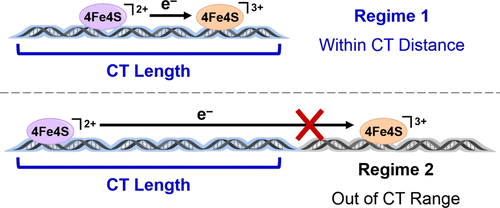
The stacked aromatic base pairs within the DNA double helix facilitate charge transport down its length in the absence of lesions, mismatches, and other stacking perturbations. DNA repair proteins containing [4Fe4S] clusters can take advantage of DNA charge transport (CT) chemistry to scan the genome for mistakes more efficiently. Here we examine the effective length over which charge can be transported along DNA between these repair proteins. We define the effective CT distance as the length of DNA within which two proteins are able to influence their ensemble affinity to the DNA duplex via CT. Endonuclease III, a DNA repair glycosylase containing a [4Fe4S] cluster, was incubated with DNA duplexes of different lengths (1.5–9 kb), and atomic force microscopy was used to quantify the binding of proteins to these duplexes to determine how the relative protein affinity changes with increasing DNA length. A sharp change in binding slope is observed at 3509 base pairs, or about 1.2 μm, that supports the existence of two regimes for protein binding, one within the range for DNA CT, one outside of the range for CT; DNA CT between the redox proteins bound to DNA effectively decreases the ensemble binding affinity of oxidized and reduced proteins to DNA. Utilizing an Endonuclease III mutant Y82A, which is defective in carrying out DNA CT, shows only one regime for protein binding. Decreasing the temperature to 4 °C or including metallointercalators on the duplex, both of which should enhance base stacking and decrease DNA floppiness, leads to extending the effective length for DNA charge transport to ∼5300 bp or 1.8 μm. These results thus support DNA charge transport between repair proteins over kilobase distances. The results furthermore highlight the ability of DNA repair proteins to search the genome quickly and efficiently using DNA charge transport chemistry.
中文翻译:

DNA介导的修复蛋白之间电荷传输的有效距离
在没有损伤,错配和其他堆积扰动的情况下,DNA双螺旋中堆积的芳族碱基对可促进电荷沿其长度向下传输。包含[4Fe4S]簇的DNA修复蛋白可以利用DNA电荷传输(CT)化学来更有效地扫描基因组中的错误。在这里,我们研究了在这些修复蛋白之间可以沿着DNA传输电荷的有效长度。我们将有效的CT距离定义为两个蛋白质能够通过CT影响其与DNA双链体的整体亲和力的DNA长度。核酸内切酶III(一种含有[4Fe4S]簇的DNA修复糖基化酶)与不同长度(1.5–9 kb)的DNA双链体一起孵育,原子力显微镜用于定量蛋白质与这些双链体的结合,以确定相对蛋白质亲和力如何随DNA长度的增加而变化。在3509个碱基对(约1.2μm)处观察到结合斜率的急剧变化,这支持存在两种蛋白质结合机制,一种在DNA CT范围内,一种在CT范围外;与DNA结合的氧化还原蛋白之间的DNA CT有效降低了氧化和还原的蛋白与DNA的整体结合亲和力。利用核酸内切酶III突变体Y82A(在进行DNA CT方面存在缺陷),仅显示了一种蛋白质结合方案。将温度降低到4°C或在双链体上包括金属嵌入剂,这两者都应增强碱基堆积并降低DNA的不稳定性,导致将DNA电荷传输的有效长度延长到〜5300 bp或1.8μm。因此,这些结果支持了修复蛋白之间在千碱基距离上的DNA电荷运输。结果进一步强调了DNA修复蛋白使用DNA电荷转移化学快速有效地搜索基因组的能力。
更新日期:2019-01-11
中文翻译:

DNA介导的修复蛋白之间电荷传输的有效距离
在没有损伤,错配和其他堆积扰动的情况下,DNA双螺旋中堆积的芳族碱基对可促进电荷沿其长度向下传输。包含[4Fe4S]簇的DNA修复蛋白可以利用DNA电荷传输(CT)化学来更有效地扫描基因组中的错误。在这里,我们研究了在这些修复蛋白之间可以沿着DNA传输电荷的有效长度。我们将有效的CT距离定义为两个蛋白质能够通过CT影响其与DNA双链体的整体亲和力的DNA长度。核酸内切酶III(一种含有[4Fe4S]簇的DNA修复糖基化酶)与不同长度(1.5–9 kb)的DNA双链体一起孵育,原子力显微镜用于定量蛋白质与这些双链体的结合,以确定相对蛋白质亲和力如何随DNA长度的增加而变化。在3509个碱基对(约1.2μm)处观察到结合斜率的急剧变化,这支持存在两种蛋白质结合机制,一种在DNA CT范围内,一种在CT范围外;与DNA结合的氧化还原蛋白之间的DNA CT有效降低了氧化和还原的蛋白与DNA的整体结合亲和力。利用核酸内切酶III突变体Y82A(在进行DNA CT方面存在缺陷),仅显示了一种蛋白质结合方案。将温度降低到4°C或在双链体上包括金属嵌入剂,这两者都应增强碱基堆积并降低DNA的不稳定性,导致将DNA电荷传输的有效长度延长到〜5300 bp或1.8μm。因此,这些结果支持了修复蛋白之间在千碱基距离上的DNA电荷运输。结果进一步强调了DNA修复蛋白使用DNA电荷转移化学快速有效地搜索基因组的能力。











































 京公网安备 11010802027423号
京公网安备 11010802027423号