Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Delta-like 1 homologue promotes tumorigenesis and epithelial-mesenchymal transition of ovarian high-grade serous carcinoma through activation of Notch signaling.
Oncogene ( IF 8 ) Pub Date : 2019-Jan-09 , DOI: 10.1038/s41388-018-0658-5 Chao-Cheng Huang , Shih-Hsuan Cheng , Chen-Hsuan Wu , Wen-Yuan Li , Jiang-Shiang Wang , Mei-Lang Kung , Tian-Huei Chu , Shih-Tsung Huang , Chien-Ting Feng , Shih-Chung Huang , Ming-Hong Tai
Oncogene ( IF 8 ) Pub Date : 2019-Jan-09 , DOI: 10.1038/s41388-018-0658-5 Chao-Cheng Huang , Shih-Hsuan Cheng , Chen-Hsuan Wu , Wen-Yuan Li , Jiang-Shiang Wang , Mei-Lang Kung , Tian-Huei Chu , Shih-Tsung Huang , Chien-Ting Feng , Shih-Chung Huang , Ming-Hong Tai
|
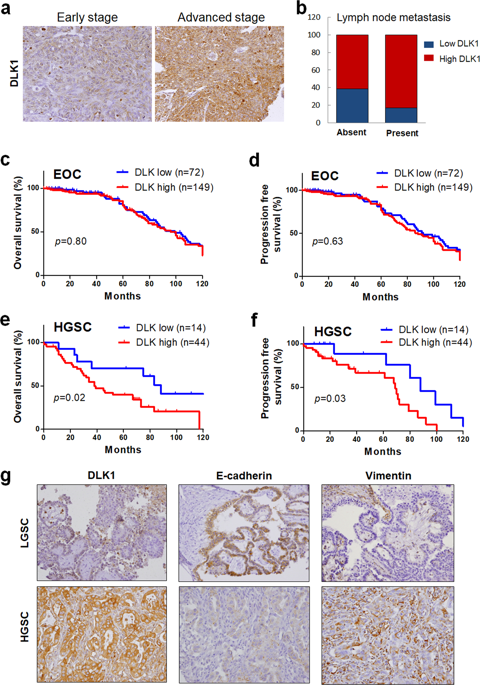
Ovarian carcinoma is the most lethal type of gynecologic malignancies. Alterations of Notch pathway are prevalent in ovarian carcinogenesis. This study investigated the expression profile and function of delta-like 1 homolog (DLK1), a non-canonical Notch ligand, during ovarian carcinogenesis. Tissue microarray (TMA) consisting of surgically resected samples from 221 patients with ovarian carcinoma was constructed for DLK1 expression. DLK1 overexpression or knockdown was achieved by adenovirus gene delivery to evaluate the effect of DLK1 on the oncogenic behaviors in ovarian cancer cells and in xenografted tumors. TMA analysis revealed that elevated DLK1 expression was correlated with stages, lymph node metastasis and E-cadherin downregulation. Despite no influence on survival among ovarian carcinoma patients, DLK1 overexpression was specially associated with overall survival and progression free survival in high-grade serous carcinoma (HGSC) patients, constituting an independent prognostic factor for these patients. By adenovirus gene delivery, it was found modulation of cellular DLK1 level regulated the tumorigenic behaviors and epithelial-mesenchymal transition (EMT) in vitro and in vivo. Immunohistochemical analysis further showed that DLK1 overexpression resulted in escalated proliferation, angiogenesis, EMT and Notch activities. Application of recombinant DLK1 extracellular domain (rDLK1-EC) recapitulated the tumorigenic behaviors of DLK1 in ovarian cancer cells. By using neutralizing antibody or pharmaceutical inhibitor, blockade of Notch signaling attenuated the tumorigenic behaviors evoked by DLK1 overexpression. The present study indicates that DLK1 overexpression participates in ovarian carcinogenesis through Notch activation and EMT induction. Moreover, DLK1 may constitute a novel diagnostic biomarker and therapeutic target for HGSC.
中文翻译:

Delta样1同源物通过激活Notch信号促进卵巢高级浆液性癌的肿瘤发生和上皮-间质转化。
卵巢癌是最致命的妇科恶性肿瘤。Notch途径的改变在卵巢癌发生中很普遍。这项研究调查了卵巢癌发生过程中非典型的Notch配体delta-like 1同源物(DLK1)的表达特征和功能。构建了由221例卵巢癌患者的手术切除样品组成的组织微阵列(TMA),用于DLK1表达。DLK1过表达或敲低是通过腺病毒基因传递来实现的,以评估DLK1对卵巢癌细胞和异种移植肿瘤中致癌行为的影响。TMA分析显示,DLK1表达升高与分期,淋巴结转移和E-cadherin下调相关。尽管对卵巢癌患者的生存没有影响,DLK1过表达与高级别浆液性癌(HGSC)患者的总体生存和无进展生存特别相关,构成了这些患者的独立预后因素。通过腺病毒基因递送,发现细胞DLK1水平的调节在体外和体内调节致瘤行为和上皮-间质转化(EMT)。免疫组织化学分析进一步表明,DLK1过表达导致增殖,血管生成,EMT和Notch活性升高。重组DLK1细胞外结构域(rDLK1-EC)的应用概述了DLK1在卵巢癌细胞中的致瘤行为。通过使用中和抗体或药物抑制剂,Notch信号的阻断减弱了DLK1过表达引起的致瘤行为。本研究表明DLK1过表达通过Notch激活和EMT诱导参与卵巢癌的发生。此外,DLK1可能构成HGSC的新型诊断生物标志物和治疗靶标。
更新日期:2019-01-26
中文翻译:

Delta样1同源物通过激活Notch信号促进卵巢高级浆液性癌的肿瘤发生和上皮-间质转化。
卵巢癌是最致命的妇科恶性肿瘤。Notch途径的改变在卵巢癌发生中很普遍。这项研究调查了卵巢癌发生过程中非典型的Notch配体delta-like 1同源物(DLK1)的表达特征和功能。构建了由221例卵巢癌患者的手术切除样品组成的组织微阵列(TMA),用于DLK1表达。DLK1过表达或敲低是通过腺病毒基因传递来实现的,以评估DLK1对卵巢癌细胞和异种移植肿瘤中致癌行为的影响。TMA分析显示,DLK1表达升高与分期,淋巴结转移和E-cadherin下调相关。尽管对卵巢癌患者的生存没有影响,DLK1过表达与高级别浆液性癌(HGSC)患者的总体生存和无进展生存特别相关,构成了这些患者的独立预后因素。通过腺病毒基因递送,发现细胞DLK1水平的调节在体外和体内调节致瘤行为和上皮-间质转化(EMT)。免疫组织化学分析进一步表明,DLK1过表达导致增殖,血管生成,EMT和Notch活性升高。重组DLK1细胞外结构域(rDLK1-EC)的应用概述了DLK1在卵巢癌细胞中的致瘤行为。通过使用中和抗体或药物抑制剂,Notch信号的阻断减弱了DLK1过表达引起的致瘤行为。本研究表明DLK1过表达通过Notch激活和EMT诱导参与卵巢癌的发生。此外,DLK1可能构成HGSC的新型诊断生物标志物和治疗靶标。



























 京公网安备 11010802027423号
京公网安备 11010802027423号