当前位置:
X-MOL 学术
›
ACS Cent. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
An ATP-Regulated Ion Transport Nanosystem for Homeostatic Perturbation Therapy and Sensitizing Photodynamic Therapy by Autophagy Inhibition of Tumors
ACS Central Science ( IF 12.7 ) Pub Date : 2019-01-08 00:00:00 , DOI: 10.1021/acscentsci.8b00822 Shuang-Shuang Wan 1 , Lu Zhang 1 , Xian-Zheng Zhang 1
ACS Central Science ( IF 12.7 ) Pub Date : 2019-01-08 00:00:00 , DOI: 10.1021/acscentsci.8b00822 Shuang-Shuang Wan 1 , Lu Zhang 1 , Xian-Zheng Zhang 1
Affiliation
|
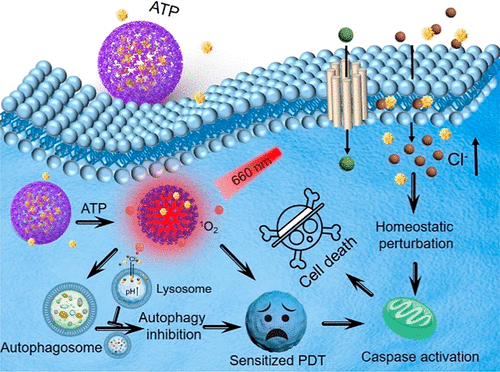
In this article, an adenosine-triphosphate-regulated (ATP-regulated) ion transport nanosystem [[email protected], porphyrinic porous coordination network (PCN) incorporated with squaramide (SQU)] was designed and synthesized for homeostatic perturbation therapy (HPT) and sensitizing photodynamic therapy (PDT) of tumors. It was found that this nanotransporter [email protected] easily accumulated in tumor sites while avoiding metabolic clearance and side effects. In response to a high expression of ATP in the tumor, [email protected] was decomposed because of the strong coordination of ATP with metal ligand of PCN. Subsequently, incorporated SQU was released and then simultaneously transported chloride ions across membrane of the cell and lysosome along with the chloride ion concentration gradient. On one hand, influx of chloride ions by SQU increased intracellular ion concentration, which disrupted ion homeostasis and further induced tumor cell apoptosis. On the other hand, SQU-medicated coupling transport of H+/Cl– across the lysosomal membrane alkalized the lysosome, resulting in inhibition of autophagy. This SQU-mediated autophagy inhibition would sensitize PCN-based PDT since activated autophagy by traditional PDT would resist and weaken the therapeutic efficacy. In vivo animal test results revealed that combined HPT and sensitized PDT could realize tumor eradication while blocking metastasis, which provided a paradigm for complementary multimodal tumor treatment.
中文翻译:

ATP 调节的离子传输纳米系统,用于稳态扰动疗法和通过抑制肿瘤自噬进行敏化光动力疗法
在本文中,设计并合成了一种三磷酸腺苷调节(ATP 调节)离子传输纳米系统[[email protected],卟啉多孔配位网络(PCN)与方酰胺(SQU)结合)用于稳态扰动疗法(HPT)和肿瘤的敏化光动力疗法(PDT)。研究发现,这种纳米转运蛋白很容易在肿瘤部位积聚,同时避免了代谢清除和副作用。响应肿瘤中 ATP 的高表达,由于 ATP 与 PCN 金属配体的强烈配位作用,[email protected] 被分解。随后,掺入的 SQU 被释放,然后随着氯离子浓度梯度同时将氯离子转运穿过细胞膜和溶酶体。一方面,SQU流入的氯离子增加了细胞内离子浓度,从而破坏了离子稳态并进一步诱导肿瘤细胞凋亡。另一方面,SQU 介导的 H + /Cl –跨溶酶体膜的偶联转运使溶酶体碱化,从而抑制自噬。这种 SQU 介导的自噬抑制将使基于 PCN 的 PDT 变得敏感,因为传统 PDT 激活的自噬会抵抗并削弱治疗效果。体内动物试验结果表明,联合HPT和敏化PDT可以实现根除肿瘤,同时阻断转移,这为互补的多模式肿瘤治疗提供了范例。
更新日期:2019-01-08
中文翻译:

ATP 调节的离子传输纳米系统,用于稳态扰动疗法和通过抑制肿瘤自噬进行敏化光动力疗法
在本文中,设计并合成了一种三磷酸腺苷调节(ATP 调节)离子传输纳米系统[[email protected],卟啉多孔配位网络(PCN)与方酰胺(SQU)结合)用于稳态扰动疗法(HPT)和肿瘤的敏化光动力疗法(PDT)。研究发现,这种纳米转运蛋白很容易在肿瘤部位积聚,同时避免了代谢清除和副作用。响应肿瘤中 ATP 的高表达,由于 ATP 与 PCN 金属配体的强烈配位作用,[email protected] 被分解。随后,掺入的 SQU 被释放,然后随着氯离子浓度梯度同时将氯离子转运穿过细胞膜和溶酶体。一方面,SQU流入的氯离子增加了细胞内离子浓度,从而破坏了离子稳态并进一步诱导肿瘤细胞凋亡。另一方面,SQU 介导的 H + /Cl –跨溶酶体膜的偶联转运使溶酶体碱化,从而抑制自噬。这种 SQU 介导的自噬抑制将使基于 PCN 的 PDT 变得敏感,因为传统 PDT 激活的自噬会抵抗并削弱治疗效果。体内动物试验结果表明,联合HPT和敏化PDT可以实现根除肿瘤,同时阻断转移,这为互补的多模式肿瘤治疗提供了范例。











































 京公网安备 11010802027423号
京公网安备 11010802027423号