当前位置:
X-MOL 学术
›
ACS Cent. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The Importance of Salt-Enhanced Electrostatic Repulsion in Colloidal Crystal Engineering with DNA
ACS Central Science ( IF 12.7 ) Pub Date : 2019-01-08 00:00:00 , DOI: 10.1021/acscentsci.8b00826 Soyoung E. Seo , Martin Girard , Monica Olvera de la Cruz , Chad A. Mirkin
ACS Central Science ( IF 12.7 ) Pub Date : 2019-01-08 00:00:00 , DOI: 10.1021/acscentsci.8b00826 Soyoung E. Seo , Martin Girard , Monica Olvera de la Cruz , Chad A. Mirkin
|
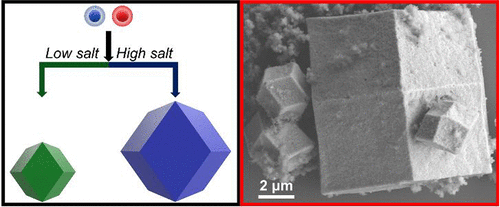
Realizing functional colloidal single crystals requires precise control over nanoparticles in three dimensions across multiple size regimes. In this regard, colloidal crystallization with programmable atom equivalents (PAEs) composed of DNA-modified nanoparticles allows one to program in a sequence-specific manner crystal symmetry, lattice parameter, and, in certain cases, crystal habit. Here, we explore how salt and the electrostatic properties of DNA regulate the attachment kinetics between PAEs. Counterintuitively, simulations and theory show that at high salt concentrations (1 M NaCl), the energy barrier for crystal growth increases by over an order of magnitude compared to low concentration (0.3 M), resulting in a transition from interface-limited to diffusion-limited crystal growth at larger crystal sizes. Remarkably, at elevated salt concentrations, well-formed rhombic dodecahedron-shaped microcrystals up to 21 μm in size grow, whereas at low salt concentration, the crystal size typically does not exceed 2 μm. Simulations show an increased barrier to hybridization between complementary PAEs at elevated salt concentrations. Therefore, although one might intuitively conclude that higher salt concentration would lead to less electrostatic repulsion and faster PAE-to-PAE hybridization kinetics, the opposite is the case, especially at larger inter-PAE distances. These observations provide important insight into how solution ionic strength can be used to control the attachment kinetics of nanoparticles coated with charged polymeric materials in general and DNA in particular.
中文翻译:

DNA胶体晶体工程中盐增强静电排斥的重要性
实现功能性胶体单晶需要在多种尺寸范围内对三维纳米粒子进行精确控制。在这方面,具有由DNA修饰的纳米粒子组成的可编程原子当量(PAE)的胶体结晶允许人们以序列特定的方式编程晶体对称性,晶格参数,在某些情况下还可以编程习惯。在这里,我们探索盐和DNA的静电特性如何调节PAE之间的附着动力学。违反直觉的是,模拟和理论表明,在高盐浓度(1 M NaCl)下,与低浓度(0.3 M)相比,晶体生长的能垒增加了一个数量级,从而导致了从界面受限到扩散的转变。较大晶体尺寸时晶体生长受到限制。值得注意的是 在较高的盐浓度下,会形成尺寸最大为21μm的结构良好的菱形十二面体形微晶,而在较低的盐浓度下,晶体尺寸通常不会超过2μm。模拟表明,在盐浓度升高的情况下,互补型PAE之间杂交的障碍增加了。因此,尽管可以直观地得出结论,较高的盐浓度将导致较少的静电排斥力和较快的PAE到PAE杂交动力学,但情况恰恰相反,尤其是在较大的PAE间距离时。这些观察结果提供了对于溶液离子强度如何可用来控制通常被带电荷的聚合物材料尤其是DNA包覆的纳米颗粒的附着动力学的重要见解。而在低盐浓度下,晶体尺寸通常不超过2μm。模拟表明,在盐浓度升高的情况下,互补型PAE之间杂交的障碍增加了。因此,尽管可以直观地得出结论,较高的盐浓度将导致较少的静电排斥力和较快的PAE到PAE杂交动力学,但情况恰恰相反,尤其是在较大的PAE间距离时。这些观察结果提供了对于溶液离子强度如何可用来控制通常被带电荷的聚合物材料尤其是DNA包覆的纳米颗粒的附着动力学的重要见解。而在低盐浓度下,晶体尺寸通常不超过2μm。模拟表明,在盐浓度升高的情况下,互补型PAE之间杂交的障碍增加了。因此,尽管可以直观地得出结论,较高的盐浓度将导致较少的静电排斥力和较快的PAE到PAE杂交动力学,但情况恰恰相反,尤其是在较大的PAE间距离时。这些观察结果提供了对于溶液离子强度如何可用来控制通常被带电荷的聚合物材料尤其是DNA包覆的纳米颗粒的附着动力学的重要见解。尽管可以直观地得出结论,较高的盐浓度将导致较少的静电排斥力和较快的PAE到PAE杂交动力学,但情况恰恰相反,尤其是在较大的PAE间距离时。这些观察结果提供了对于溶液离子强度如何可用来控制通常被带电荷的聚合物材料尤其是DNA包覆的纳米颗粒的附着动力学的重要见解。尽管可以直观地得出结论,较高的盐浓度将导致较少的静电排斥力和较快的PAE到PAE杂交动力学,但情况恰恰相反,尤其是在较大的PAE间距离时。这些观察结果提供了对于溶液离子强度如何可用来控制通常被带电荷的聚合物材料尤其是DNA包覆的纳米颗粒的附着动力学的重要见解。
更新日期:2019-01-08
中文翻译:

DNA胶体晶体工程中盐增强静电排斥的重要性
实现功能性胶体单晶需要在多种尺寸范围内对三维纳米粒子进行精确控制。在这方面,具有由DNA修饰的纳米粒子组成的可编程原子当量(PAE)的胶体结晶允许人们以序列特定的方式编程晶体对称性,晶格参数,在某些情况下还可以编程习惯。在这里,我们探索盐和DNA的静电特性如何调节PAE之间的附着动力学。违反直觉的是,模拟和理论表明,在高盐浓度(1 M NaCl)下,与低浓度(0.3 M)相比,晶体生长的能垒增加了一个数量级,从而导致了从界面受限到扩散的转变。较大晶体尺寸时晶体生长受到限制。值得注意的是 在较高的盐浓度下,会形成尺寸最大为21μm的结构良好的菱形十二面体形微晶,而在较低的盐浓度下,晶体尺寸通常不会超过2μm。模拟表明,在盐浓度升高的情况下,互补型PAE之间杂交的障碍增加了。因此,尽管可以直观地得出结论,较高的盐浓度将导致较少的静电排斥力和较快的PAE到PAE杂交动力学,但情况恰恰相反,尤其是在较大的PAE间距离时。这些观察结果提供了对于溶液离子强度如何可用来控制通常被带电荷的聚合物材料尤其是DNA包覆的纳米颗粒的附着动力学的重要见解。而在低盐浓度下,晶体尺寸通常不超过2μm。模拟表明,在盐浓度升高的情况下,互补型PAE之间杂交的障碍增加了。因此,尽管可以直观地得出结论,较高的盐浓度将导致较少的静电排斥力和较快的PAE到PAE杂交动力学,但情况恰恰相反,尤其是在较大的PAE间距离时。这些观察结果提供了对于溶液离子强度如何可用来控制通常被带电荷的聚合物材料尤其是DNA包覆的纳米颗粒的附着动力学的重要见解。而在低盐浓度下,晶体尺寸通常不超过2μm。模拟表明,在盐浓度升高的情况下,互补型PAE之间杂交的障碍增加了。因此,尽管可以直观地得出结论,较高的盐浓度将导致较少的静电排斥力和较快的PAE到PAE杂交动力学,但情况恰恰相反,尤其是在较大的PAE间距离时。这些观察结果提供了对于溶液离子强度如何可用来控制通常被带电荷的聚合物材料尤其是DNA包覆的纳米颗粒的附着动力学的重要见解。尽管可以直观地得出结论,较高的盐浓度将导致较少的静电排斥力和较快的PAE到PAE杂交动力学,但情况恰恰相反,尤其是在较大的PAE间距离时。这些观察结果提供了对于溶液离子强度如何可用来控制通常被带电荷的聚合物材料尤其是DNA包覆的纳米颗粒的附着动力学的重要见解。尽管可以直观地得出结论,较高的盐浓度将导致较少的静电排斥力和较快的PAE到PAE杂交动力学,但情况恰恰相反,尤其是在较大的PAE间距离时。这些观察结果提供了对于溶液离子强度如何可用来控制通常被带电荷的聚合物材料尤其是DNA包覆的纳米颗粒的附着动力学的重要见解。











































 京公网安备 11010802027423号
京公网安备 11010802027423号