当前位置:
X-MOL 学术
›
Chem. Rev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Intracellular Catalysis with Selected Metal Complexes and Metallic Nanoparticles: Advances toward the Development of Catalytic Metallodrugs
Chemical Reviews ( IF 51.4 ) Pub Date : 2019-01-08 00:00:00 , DOI: 10.1021/acs.chemrev.8b00493 Joan Josep Soldevila-Barreda 1 , Nils Metzler-Nolte 1
Chemical Reviews ( IF 51.4 ) Pub Date : 2019-01-08 00:00:00 , DOI: 10.1021/acs.chemrev.8b00493 Joan Josep Soldevila-Barreda 1 , Nils Metzler-Nolte 1
Affiliation
|
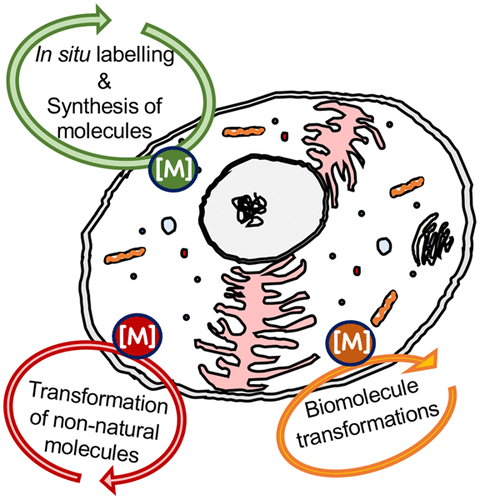
Platinum-containing drugs (e.g., cisplatin) are among the most frequently used chemotherapeutic agents. Their tremendous success has spurred research and development of other metal-based drugs, with notable achievements. Generally, the vast majority of metal-based drug candidates in clinical and developmental stages are stoichiometric agents, i.e., each metal complex reacts only once with their biological target. Additionally, many of these metal complexes are involved in side reactions, which not only reduce the effective amount of the drug but may also cause toxicity. On a separate note, transition metal complexes and nanoparticles have a well-established history of being potent catalysts for selective molecular transformations, with examples such as the Mo- and Ru-based catalysts for metathesis reactions (Nobel Prize in 2005) or palladium catalysts for C–C bond forming reactions such as Heck, Negishi, or Suzuki reactions (Nobel Prize in 2010). Also, notably, no direct biological equivalent of these transformations exists in a biological environment such as bacteria or mammalian cells. It is, therefore, only logical that recent interest has focused on developing transition-metal based catalytic systems that are capable of performing transformations inside cells, with the aim of inducing medicinally relevant cellular changes. Because unlike in stoichiometric reactions, a catalytically active compound may turn over many substrate molecules, only very small amounts of such a catalytic metallodrug are required to achieve a desired pharmacologic effect, and therefore, toxicity and side reactions are reduced. Furthermore, performing catalytic reactions in biological systems also opens the door for new methodologies to study the behavior of biomolecules in their natural state, e.g., via in situ labeling or by increasing/depleting their concentration at will. There is, of course, an art to the choice of catalysts and reactions which have to be compatible with biological conditions, namely an aqueous, oxygen-containing environment. In this review, we aim to describe new developments that bring together the far-distant worlds of transition-metal based catalysis and metal-based drugs, in what is termed “catalytic metallodrugs”. Here we will focus on transformations that have been performed on small biomolecules (such as shifting equilibria like in the NAD+/NADH or GSH/GSSG couples), on non-natural molecules such as dyes for imaging purposes, or on biomacromolecules such as proteins. Neither reactions involving release (e.g., CO) or transformation of small molecules (e.g., 1O2 production), degradation of biomolecules such as proteins, RNA or DNA nor light-induced medicinal chemistry (e.g., photodynamic therapy) are covered, even if metal complexes are centrally involved in those. In each section, we describe the (inorganic) chemistry involved, as well as selected examples of biological applications in the hope that this snapshot of a new but quickly developing field will indeed inspire novel research and unprecedented interactions across disciplinary boundaries.
中文翻译:

选择性金属配合物和金属纳米粒子的细胞内催化:催化金属药物发展的进展。
含铂药物(例如,顺铂)是最常用的化学治疗剂。他们的巨大成功刺激了其他金属基药物的研发,并取得了显著成就。通常,在临床和开发阶段,绝大多数基于金属的候选药物是化学计量试剂,即每种金属配合物与其生物学靶标仅反应一次。另外,许多这些金属络合物参与副反应,这不仅降低了药物的有效量,而且还可能引起毒性。另外,过渡金属络合物和纳米颗粒具有悠久的历史,可以作为选择性分子转化的有效催化剂,举例来说,用于置换反应的Mo和Ru基催化剂(2005年诺贝尔奖)或用于C–C键形成反应的钯催化剂(例如Heck,Negishi或Suzuki反应)(2010年诺贝尔奖)。同样,值得注意的是,在诸如细菌或哺乳动物细胞的生物环境中,不存在这些转化的直接生物学等同物。因此,合乎逻辑的是,最近的兴趣集中在开发基于过渡金属的催化系统上,该催化系统能够在细胞内进行转化,以诱导医学上相关的细胞变化。因为与化学计量反应不同,催化活性化合物可能会转换许多底物分子,因此仅需要非常少量的这种催化金属药物即可达到所需的药理作用,因此,减少毒性和副反应。此外,在生物系统中进行催化反应也为研究自然状态下生物分子行为的新方法打开了大门,例如通过原位标记或随意增加/减少其浓度。当然,选择与生物条件(即含水的含氧环境)相容的催化剂和反应是本领域的技术。在这篇综述中,我们旨在描述将基于过渡金属的催化和基于金属的药物(称为“催化金属药物”)的遥远世界融合在一起的新进展。在这里,我们将重点介绍对小生物分子进行的转化(例如,NAD中的平衡转移)在生物系统中进行催化反应也为研究自然状态下生物分子行为的新方法打开了大门,例如通过原位标记或随意增加/减少其浓度。当然,选择与生物条件(即含水的含氧环境)相容的催化剂和反应是本领域的技术。在这篇综述中,我们旨在描述将基于过渡金属的催化和基于金属的药物(称为“催化金属药物”)的遥远世界融合在一起的新进展。在这里,我们将重点介绍对小生物分子进行的转化(例如,NAD中的平衡转移)在生物系统中进行催化反应也为研究自然状态下生物分子行为的新方法打开了大门,例如通过原位标记或随意增加/减少其浓度。当然,选择与生物条件(即含水的含氧环境)相容的催化剂和反应是本领域的技术。在这篇综述中,我们旨在描述将基于过渡金属的催化和基于金属的药物(称为“催化金属药物”)的遥远世界融合在一起的新进展。在这里,我们将重点介绍对小生物分子进行的转化(例如,NAD中的平衡转移)选择催化剂和反应的技术是必须与生物学条件,即含水的含氧环境相容的。在这篇综述中,我们旨在描述将基于过渡金属的催化和基于金属的药物(称为“催化金属药物”)的遥远世界融合在一起的新进展。在这里,我们将重点介绍对小生物分子进行的转化(例如,NAD中的平衡转移)选择催化剂和反应的技术是必须与生物学条件,即含水的含氧环境相容的。在这篇综述中,我们旨在描述将基于过渡金属的催化和基于金属的药物(称为“催化金属药物”)的遥远世界融合在一起的新进展。在这里,我们将重点介绍对小生物分子进行的转化(例如,NAD中的平衡转移)+ / NADH或GSH / GSSG对),在非天然分子(例如用于成像目的的染料)上,或在生物大分子(例如蛋白质)上。即使涉及释放(例如,CO)或小分子转化(例如,产生1 O 2),生物分子(例如蛋白质,RNA或DNA)降解或光诱导药物化学(例如,光动力疗法)的反应均未涵盖,即使金属配合物主要参与其中。在每个部分中,我们都将描述所涉及的(无机)化学以及生物学应用的一些示例,以期这个新的但迅速发展的领域的快照确实将激发新颖的研究和跨学科界限的前所未有的相互作用。
更新日期:2019-01-08
中文翻译:

选择性金属配合物和金属纳米粒子的细胞内催化:催化金属药物发展的进展。
含铂药物(例如,顺铂)是最常用的化学治疗剂。他们的巨大成功刺激了其他金属基药物的研发,并取得了显著成就。通常,在临床和开发阶段,绝大多数基于金属的候选药物是化学计量试剂,即每种金属配合物与其生物学靶标仅反应一次。另外,许多这些金属络合物参与副反应,这不仅降低了药物的有效量,而且还可能引起毒性。另外,过渡金属络合物和纳米颗粒具有悠久的历史,可以作为选择性分子转化的有效催化剂,举例来说,用于置换反应的Mo和Ru基催化剂(2005年诺贝尔奖)或用于C–C键形成反应的钯催化剂(例如Heck,Negishi或Suzuki反应)(2010年诺贝尔奖)。同样,值得注意的是,在诸如细菌或哺乳动物细胞的生物环境中,不存在这些转化的直接生物学等同物。因此,合乎逻辑的是,最近的兴趣集中在开发基于过渡金属的催化系统上,该催化系统能够在细胞内进行转化,以诱导医学上相关的细胞变化。因为与化学计量反应不同,催化活性化合物可能会转换许多底物分子,因此仅需要非常少量的这种催化金属药物即可达到所需的药理作用,因此,减少毒性和副反应。此外,在生物系统中进行催化反应也为研究自然状态下生物分子行为的新方法打开了大门,例如通过原位标记或随意增加/减少其浓度。当然,选择与生物条件(即含水的含氧环境)相容的催化剂和反应是本领域的技术。在这篇综述中,我们旨在描述将基于过渡金属的催化和基于金属的药物(称为“催化金属药物”)的遥远世界融合在一起的新进展。在这里,我们将重点介绍对小生物分子进行的转化(例如,NAD中的平衡转移)在生物系统中进行催化反应也为研究自然状态下生物分子行为的新方法打开了大门,例如通过原位标记或随意增加/减少其浓度。当然,选择与生物条件(即含水的含氧环境)相容的催化剂和反应是本领域的技术。在这篇综述中,我们旨在描述将基于过渡金属的催化和基于金属的药物(称为“催化金属药物”)的遥远世界融合在一起的新进展。在这里,我们将重点介绍对小生物分子进行的转化(例如,NAD中的平衡转移)在生物系统中进行催化反应也为研究自然状态下生物分子行为的新方法打开了大门,例如通过原位标记或随意增加/减少其浓度。当然,选择与生物条件(即含水的含氧环境)相容的催化剂和反应是本领域的技术。在这篇综述中,我们旨在描述将基于过渡金属的催化和基于金属的药物(称为“催化金属药物”)的遥远世界融合在一起的新进展。在这里,我们将重点介绍对小生物分子进行的转化(例如,NAD中的平衡转移)选择催化剂和反应的技术是必须与生物学条件,即含水的含氧环境相容的。在这篇综述中,我们旨在描述将基于过渡金属的催化和基于金属的药物(称为“催化金属药物”)的遥远世界融合在一起的新进展。在这里,我们将重点介绍对小生物分子进行的转化(例如,NAD中的平衡转移)选择催化剂和反应的技术是必须与生物学条件,即含水的含氧环境相容的。在这篇综述中,我们旨在描述将基于过渡金属的催化和基于金属的药物(称为“催化金属药物”)的遥远世界融合在一起的新进展。在这里,我们将重点介绍对小生物分子进行的转化(例如,NAD中的平衡转移)+ / NADH或GSH / GSSG对),在非天然分子(例如用于成像目的的染料)上,或在生物大分子(例如蛋白质)上。即使涉及释放(例如,CO)或小分子转化(例如,产生1 O 2),生物分子(例如蛋白质,RNA或DNA)降解或光诱导药物化学(例如,光动力疗法)的反应均未涵盖,即使金属配合物主要参与其中。在每个部分中,我们都将描述所涉及的(无机)化学以及生物学应用的一些示例,以期这个新的但迅速发展的领域的快照确实将激发新颖的研究和跨学科界限的前所未有的相互作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号