当前位置:
X-MOL 学术
›
ACS Cent. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Lipid-Dependent Alternating Access Mechanism of a Bacterial Multidrug ABC Exporter
ACS Central Science ( IF 12.7 ) Pub Date : 2019-01-07 00:00:00 , DOI: 10.1021/acscentsci.8b00480 Kalyan Immadisetty 1 , Jeevapani Hettige 1 , Mahmoud Moradi 1
ACS Central Science ( IF 12.7 ) Pub Date : 2019-01-07 00:00:00 , DOI: 10.1021/acscentsci.8b00480 Kalyan Immadisetty 1 , Jeevapani Hettige 1 , Mahmoud Moradi 1
Affiliation
|
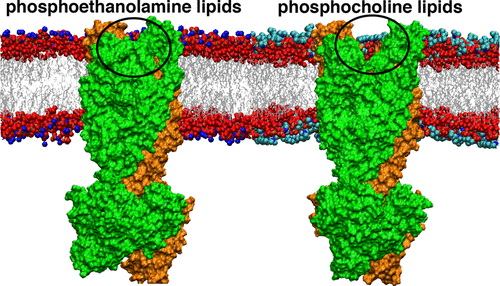
By undergoing conformational changes, active membrane transporters alternate between an inward-facing (IF) and an outward-facing (OF) state to transport their substrates across cellular membrane. The conformational landscape of membrane transporters, however, could be influenced by their environment, and the dependence of the alternating access mechanism on the lipid composition has not been understood at the molecular level. We have performed an extensive set of microsecond-level all-atom molecular dynamics (MD) simulations on bacterial ATP binding cassette (ABC) exporter Sav1866 in six different phosphocholine (PC) and phosphoethanolamine (PE) lipid membrane environments. This study mainly focuses on the energetically downhill OF-to-IF conformational transition of Sav1866 upon the ATP hydrolysis. We observe that the transporter undergoes large-scale conformational changes in the PE environment, particularly in the POPE lipids, resulting in an IF-occluded conformation, a transition that does not occur when the transporter is embedded in any of the PC lipid bilayers. We propose that the PE lipids facilitate the closing of the protein on the periplasmic side due to their highly polar headgroups that mediate the interaction of the two transmembrane (TM) bundles by a network of lipid–lipid and lipid–protein hydrogen bonds. POPE lipids in particular facilitate the closure of periplasmic gate by promoting a hinge formation in TM helices and an interbundle salt bridge formation. This study explains how the alternating access mechanism and the flippase activity in ABC exporters could be lipid-dependent.
中文翻译:

细菌多药ABC出口商的脂质依赖性交替访问机制
通过进行构象变化,活性膜转运蛋白在向内(IF)和朝外(OF)状态之间交替,以将其底物转运通过细胞膜。然而,膜转运蛋白的构象景观可能会受到其环境的影响,并且在分子水平上还不清楚交替进入机制对脂质组成的依赖性。我们已经在六种不同的磷胆碱(PC)和磷乙醇胺(PE)脂质膜环境中,对细菌ATP结合盒(ABC)出口商Sav1866进行了广泛的微秒级全原子分子动力学(MD)模拟。这项研究主要侧重于ATP水解后Sav1866的能量下坡从OF到IF的构象转变。我们观察到转运蛋白在PE环境中,特别是在POPE脂质中经历了大规模的构象变化,导致IF阻塞的构象,当转运蛋白嵌入任何PC脂质双层中时,不会发生这种转变。我们认为,PE脂质由于其极性极高的头基通过脂质-脂质和脂质-蛋白质氢键网络介导两个跨膜(TM)束的相互作用,因此促进了质膜侧蛋白质的封闭。POPE脂质特别通过促进TM螺旋中的铰链形成和束间盐桥形成,促进了周质门的关闭。这项研究解释了ABC出口商的交替进入机制和脂肪酶活性如何可能是脂质依赖性的。
更新日期:2019-01-07
中文翻译:

细菌多药ABC出口商的脂质依赖性交替访问机制
通过进行构象变化,活性膜转运蛋白在向内(IF)和朝外(OF)状态之间交替,以将其底物转运通过细胞膜。然而,膜转运蛋白的构象景观可能会受到其环境的影响,并且在分子水平上还不清楚交替进入机制对脂质组成的依赖性。我们已经在六种不同的磷胆碱(PC)和磷乙醇胺(PE)脂质膜环境中,对细菌ATP结合盒(ABC)出口商Sav1866进行了广泛的微秒级全原子分子动力学(MD)模拟。这项研究主要侧重于ATP水解后Sav1866的能量下坡从OF到IF的构象转变。我们观察到转运蛋白在PE环境中,特别是在POPE脂质中经历了大规模的构象变化,导致IF阻塞的构象,当转运蛋白嵌入任何PC脂质双层中时,不会发生这种转变。我们认为,PE脂质由于其极性极高的头基通过脂质-脂质和脂质-蛋白质氢键网络介导两个跨膜(TM)束的相互作用,因此促进了质膜侧蛋白质的封闭。POPE脂质特别通过促进TM螺旋中的铰链形成和束间盐桥形成,促进了周质门的关闭。这项研究解释了ABC出口商的交替进入机制和脂肪酶活性如何可能是脂质依赖性的。











































 京公网安备 11010802027423号
京公网安备 11010802027423号