当前位置:
X-MOL 学术
›
Energy Environ. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Direct observation of active catalyst surface phases and the effect of dynamic self-optimization in NiFe-layered double hydroxides for alkaline water splitting†
Energy & Environmental Science ( IF 32.4 ) Pub Date : 2019-01-04 00:00:00 , DOI: 10.1039/c8ee03282c Zhen Qiu 1, 2, 3, 4, 5 , Cheuk-Wai Tai 5, 6, 7, 8 , Gunnar A. Niklasson 1, 2, 3, 4, 5 , Tomas Edvinsson 1, 2, 3, 4, 5
Energy & Environmental Science ( IF 32.4 ) Pub Date : 2019-01-04 00:00:00 , DOI: 10.1039/c8ee03282c Zhen Qiu 1, 2, 3, 4, 5 , Cheuk-Wai Tai 5, 6, 7, 8 , Gunnar A. Niklasson 1, 2, 3, 4, 5 , Tomas Edvinsson 1, 2, 3, 4, 5
Affiliation
|
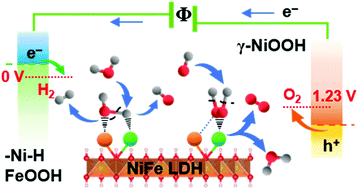
Earth-abundant transition metal-based compounds are of high interest as catalysts for sustainable hydrogen fuel generation. The realization of effective electrolysis of water, however, is still limited by the requirement of a high sustainable driving potential above thermodynamic requirements. Here, we report dynamically self-optimized (DSO) NiFe layered double hydroxide (LDH) nanosheets with promising bi-functional performance. Compared with pristine NiFe LDH, DSO NiFe LDH exhibits much lower overpotential for the hydrogen evolution reaction (HER), even outperforming platinum. Under 1 M KOH aqueous electrolyte, the bi-functional DSO catalysts show an overpotential of 184 and −59 mV without iR compensation for oxygen evolution reaction (OER) and HER at 10 mA cm−2. The material system operates at 1.48 V and 1.29 V to reach 10 and 1 mA cm−2 in two-electrode measurements, corresponding to 83% and 95% electricity-to-fuel conversion efficiency with respect to the lower heating value of hydrogen. The material is seen to dynamically reform the active phase of the surface layer during HER and OER, where the pristine and activated catalysts are analyzed with ex situ XPS, SAED and EELS as well as with in situ Raman spectro-electrochemistry. The results show transformation into different active interfacial species during OER and HER, revealing a synergistic interplay between iron and nickel in facilitating water electrolysis.
中文翻译:

直接观察活性催化剂表面相和NiFe层状双氢氧化物用于碱水分解的动态自我优化效果†
富含地球的过渡金属基化合物作为可持续氢燃料生产的催化剂备受关注。然而,水的有效电解的实现仍然受到高于热力学要求的高可持续驱动电位的要求的限制。在这里,我们报道了具有前途双功能性能的动态自优化(DSO)NiFe层状双氢氧化物(LDH)纳米片。与原始NiFe LDH相比,DSO NiFe LDH的放氢反应(HER)表现出低得多的超电势,甚至胜过铂。在1 M KOH水性电解质下,双功能DSO催化剂在10 mA cm -2时表现出184和-59 mV的过电势,而没有氧生成反应(OER)和HER的iR补偿。。该材料系统在1.48 V和1.29 V下工作,在两电极测量中达到10 mA和1 mA cm -2,相对于较低的氢热值,相当于83%和95%的电燃料转换效率。可以看出,该材料可在HER和OER过程中动态重整表面层的活性相,在其中可以通过非原位XPS,SAED和EELS以及原位拉曼光谱电化学分析原始催化剂和活化催化剂。结果表明,在OER和HER期间转化为不同的活性界面物种,揭示了铁和镍之间的相互作用,促进了水的电解。
更新日期:2019-01-04
中文翻译:

直接观察活性催化剂表面相和NiFe层状双氢氧化物用于碱水分解的动态自我优化效果†
富含地球的过渡金属基化合物作为可持续氢燃料生产的催化剂备受关注。然而,水的有效电解的实现仍然受到高于热力学要求的高可持续驱动电位的要求的限制。在这里,我们报道了具有前途双功能性能的动态自优化(DSO)NiFe层状双氢氧化物(LDH)纳米片。与原始NiFe LDH相比,DSO NiFe LDH的放氢反应(HER)表现出低得多的超电势,甚至胜过铂。在1 M KOH水性电解质下,双功能DSO催化剂在10 mA cm -2时表现出184和-59 mV的过电势,而没有氧生成反应(OER)和HER的iR补偿。。该材料系统在1.48 V和1.29 V下工作,在两电极测量中达到10 mA和1 mA cm -2,相对于较低的氢热值,相当于83%和95%的电燃料转换效率。可以看出,该材料可在HER和OER过程中动态重整表面层的活性相,在其中可以通过非原位XPS,SAED和EELS以及原位拉曼光谱电化学分析原始催化剂和活化催化剂。结果表明,在OER和HER期间转化为不同的活性界面物种,揭示了铁和镍之间的相互作用,促进了水的电解。











































 京公网安备 11010802027423号
京公网安备 11010802027423号