当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Catalyst-Free Annulation of 2-Pyridylacetates and Ynals with Molecular Oxygen: An Access to 3-Acylated Indolizines
The Journal of Organic Chemistry ( IF 3.6 ) Pub Date : 2019-01-04 00:00:00 , DOI: 10.1021/acs.joc.8b02883 Zhengwang Chen , Pei Liang , Xiaoyue Ma , Haiqing Luo , Guohai Xu , Tanggao Liu , Xiaowei Wen , Jing Zheng , Hui Ye
The Journal of Organic Chemistry ( IF 3.6 ) Pub Date : 2019-01-04 00:00:00 , DOI: 10.1021/acs.joc.8b02883 Zhengwang Chen , Pei Liang , Xiaoyue Ma , Haiqing Luo , Guohai Xu , Tanggao Liu , Xiaowei Wen , Jing Zheng , Hui Ye
|
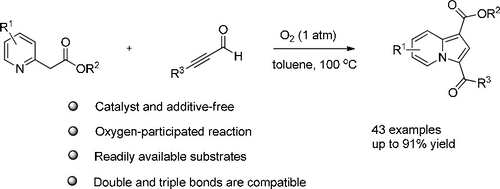
A catalyst and additive-free annulation of 2-pyridylacetates and ynals under molecular oxygen was the first developed, affording 3-acylated indolizines in good to excellent yields. Molecular oxygen was used as the source of the carbonyl oxygen atom in indolizines. This approach was compatible with a wide range of functional groups, and especially it has been successfully extended to unsaturated double bonds and triple bonds, which were difficult to prepare by previous methods in a single step.
中文翻译:

2-吡啶基乙酸酯和Ynals与分子氧的无催化剂环合:进入3-酰化吲哚嗪的途径
最早开发出了在分子氧下无催化剂的2-吡啶基乙酸酯和乙醛环化反应的方法,可提供3-酰化的吲哚嗪类化合物,收率好至极佳。分子氧被用作吲哚嗪中羰基氧原子的来源。该方法与广泛的官能团兼容,尤其是已成功地扩展到不饱和双键和三键,这是以前的方法难以在单个步骤中制备的。
更新日期:2019-01-04
中文翻译:

2-吡啶基乙酸酯和Ynals与分子氧的无催化剂环合:进入3-酰化吲哚嗪的途径
最早开发出了在分子氧下无催化剂的2-吡啶基乙酸酯和乙醛环化反应的方法,可提供3-酰化的吲哚嗪类化合物,收率好至极佳。分子氧被用作吲哚嗪中羰基氧原子的来源。该方法与广泛的官能团兼容,尤其是已成功地扩展到不饱和双键和三键,这是以前的方法难以在单个步骤中制备的。























































 京公网安备 11010802027423号
京公网安备 11010802027423号