当前位置:
X-MOL 学术
›
Mol. Pharmaceutics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Exploring the Binding Interaction Mechanism of Taxol in β-Tubulin and Bovine Serum Albumin: A Biophysical Approach
Molecular Pharmaceutics ( IF 4.5 ) Pub Date : 2019-01-02 00:00:00 , DOI: 10.1021/acs.molpharmaceut.8b00948 Subramani Karthikeyan 1, 2 , Ganesan Bharanidharan 1 , Sriram Ragavan 3 , Saravanan Kandasamy 4 , Shanmugavel Chinnathambi 5 , Kanniyappan Udayakumar 6 , Rajendiran Mangaiyarkarasi 1 , Ramakrishnamurthy Suganya 1 , Prakasarao Aruna 1 , Singaravelu Ganesan 1
Molecular Pharmaceutics ( IF 4.5 ) Pub Date : 2019-01-02 00:00:00 , DOI: 10.1021/acs.molpharmaceut.8b00948 Subramani Karthikeyan 1, 2 , Ganesan Bharanidharan 1 , Sriram Ragavan 3 , Saravanan Kandasamy 4 , Shanmugavel Chinnathambi 5 , Kanniyappan Udayakumar 6 , Rajendiran Mangaiyarkarasi 1 , Ramakrishnamurthy Suganya 1 , Prakasarao Aruna 1 , Singaravelu Ganesan 1
Affiliation
|
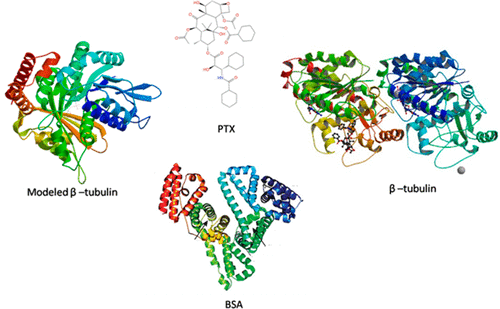
In this present study on understanding the taxol (PTX) binding interaction mechanism in both the β-tubulin and bovine serum albumin (BSA) molecule, various optical spectroscopy and computational techniques were used. The fluorescence steady-state emission spectroscopy result suggests that there is a static quenching mechanism of the PTX drug in both β-tubulin and BSA, and further time-resolved emission spectroscopy studies confirm that the quenching mechanism exists. The excitation–emission matrix (EEM), Fourier transform infrared, and resonance light scattering spectra (FT-IR) confirm that there are structural changes in both the BSA and β-tubulin molecule during the binding process of PTX. The molecular docking studies revealed the PTX binding information in BSA, β-tubulin, and modeled β-tubulin and the best binding pose to further subject the molecular dynamics simulation, and this study confirms the stability of PTX in the protein complex during the simulation. Density functional theory (DFT) calculations were performed between the free PTX drug and PTX drug (single point) in the protein molecule active site region to understand the internal stability.
中文翻译:

探索紫杉醇在β-微管蛋白和牛血清白蛋白中的结合相互作用机理:一种生物物理方法
在本研究中,为了了解紫杉醇(PTX)在β微管蛋白和牛血清白蛋白(BSA)分子中的结合相互作用机理,使用了各种光谱学和计算技术。荧光稳态发射光谱结果表明,β-微管蛋白和BSA均存在PTX药物的静态猝灭机理,进一步的时间分辨发射光谱研究证实了该猝灭机理的存在。激发发射矩阵(EEM),傅立叶变换红外光谱和共振光散射光谱(FT-IR)证实,在PTX的结合过程中,BSA和β-微管蛋白分子均发生结构变化。分子对接研究揭示了BTX,β-微管蛋白,并通过建模β-微管蛋白和最佳结合姿势进一步进行分子动力学模拟,这项研究证实了在模拟过程中PTX在蛋白质复合物中的稳定性。在蛋白质分子活性位点区域中的游离PTX药物和PTX药物(单点)之间进行了密度泛函理论(DFT)计算,以了解内部稳定性。
更新日期:2019-01-02
中文翻译:

探索紫杉醇在β-微管蛋白和牛血清白蛋白中的结合相互作用机理:一种生物物理方法
在本研究中,为了了解紫杉醇(PTX)在β微管蛋白和牛血清白蛋白(BSA)分子中的结合相互作用机理,使用了各种光谱学和计算技术。荧光稳态发射光谱结果表明,β-微管蛋白和BSA均存在PTX药物的静态猝灭机理,进一步的时间分辨发射光谱研究证实了该猝灭机理的存在。激发发射矩阵(EEM),傅立叶变换红外光谱和共振光散射光谱(FT-IR)证实,在PTX的结合过程中,BSA和β-微管蛋白分子均发生结构变化。分子对接研究揭示了BTX,β-微管蛋白,并通过建模β-微管蛋白和最佳结合姿势进一步进行分子动力学模拟,这项研究证实了在模拟过程中PTX在蛋白质复合物中的稳定性。在蛋白质分子活性位点区域中的游离PTX药物和PTX药物(单点)之间进行了密度泛函理论(DFT)计算,以了解内部稳定性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号