当前位置:
X-MOL 学术
›
ACS Chem. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
NMR Dynamics Study Reveals the Zα Domain of Human ADAR1 Associates with and Dissociates from Z-RNA More Slowly than Z-DNA.
ACS Chemical Biology ( IF 3.5 ) Pub Date : 2019-01-11 , DOI: 10.1021/acschembio.8b00914 Ae-Ree Lee 1 , Jihyun Hwang 2 , Jeong Hwan Hur 3 , Kyoung-Seok Ryu 4 , Kyeong Kyu Kim 3 , Byong-Seok Choi 2 , Nak-Kyoon Kim 5 , Joon-Hwa Lee 1
ACS Chemical Biology ( IF 3.5 ) Pub Date : 2019-01-11 , DOI: 10.1021/acschembio.8b00914 Ae-Ree Lee 1 , Jihyun Hwang 2 , Jeong Hwan Hur 3 , Kyoung-Seok Ryu 4 , Kyeong Kyu Kim 3 , Byong-Seok Choi 2 , Nak-Kyoon Kim 5 , Joon-Hwa Lee 1
Affiliation
|
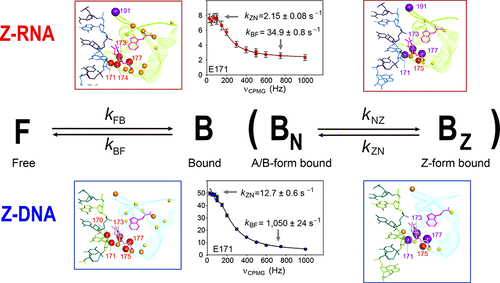
Human RNA editing enzyme ADAR1 deaminates adenosine in pre-mRNA to yield inosine. The Zα domain of human ADAR1 (hZαADAR1) binds specifically to left-handed Z-RNA as well as Z-DNA and stabilizes the Z-conformation. To answer the question of how hZαADAR1 can induce both the B-Z transition of DNA and the A-Z transition of RNA, we investigated the structure and dynamics of hZαADAR1 in complex with 6-base-pair Z-DNA or Z-RNA. We performed chemical shift perturbation and relaxation dispersion experiments on hZαADAR1 upon binding to Z-DNA as well as Z-RNA. Our study demonstrates the unique dynamics of hZαADAR1 during the A-Z transition of RNA, in which the hZαADAR1 protein forms a thermodynamically stable complex with Z-RNA, similar to Z-DNA, but kinetically converts RNA to the Z-form more slowly than DNA. We also discovered some distinct structural features of hZαADAR1 in the Z-RNA binding conformation. Our results suggest that the A-Z transition of RNA facilitated by hZαADAR1 displays unique structural and dynamic features that may be involved in targeting ADAR1 for a role in recognition of RNA substrates.
中文翻译:

NMR动力学研究揭示了人类ADAR1的Zα结构域与Z-RNA缔合和解离的速度比Z-DNA慢。
人RNA编辑酶ADAR1使pre-mRNA中的腺苷脱氨,产生肌苷。人ADAR1的Zα域(hZαADAR1)与左手Z-RNA以及Z-DNA特异性结合,并稳定Z构象。为了回答hZαADAR1如何同时诱导DNA的BZ转变和RNA的AZ转变的问题,我们研究了hZαADAR1在具有6个碱基对的Z-DNA或Z-RNA的复合物中的结构和动力学。当我们结合Z-DNA和Z-RNA时,我们在hZαADAR1上进行了化学位移扰动和弛豫分散实验。我们的研究证明了hZαADAR1在RNA的AZ过渡过程中的独特动力学,其中hZαADAR1蛋白与Z-RNA形成了热力学稳定的复合物,类似于Z-DNA,但在动力学上将RNA转化为Z形式的速度比DNA慢。我们还发现了ZZ-RNA结合构象中hZαADAR1的一些独特的结构特征。我们的结果表明,由hZαADAR1促进的RNA的AZ过渡表现出独特的结构和动态特征,可能与靶向ADAR1参与识别RNA底物有关。
更新日期:2018-12-28
中文翻译:

NMR动力学研究揭示了人类ADAR1的Zα结构域与Z-RNA缔合和解离的速度比Z-DNA慢。
人RNA编辑酶ADAR1使pre-mRNA中的腺苷脱氨,产生肌苷。人ADAR1的Zα域(hZαADAR1)与左手Z-RNA以及Z-DNA特异性结合,并稳定Z构象。为了回答hZαADAR1如何同时诱导DNA的BZ转变和RNA的AZ转变的问题,我们研究了hZαADAR1在具有6个碱基对的Z-DNA或Z-RNA的复合物中的结构和动力学。当我们结合Z-DNA和Z-RNA时,我们在hZαADAR1上进行了化学位移扰动和弛豫分散实验。我们的研究证明了hZαADAR1在RNA的AZ过渡过程中的独特动力学,其中hZαADAR1蛋白与Z-RNA形成了热力学稳定的复合物,类似于Z-DNA,但在动力学上将RNA转化为Z形式的速度比DNA慢。我们还发现了ZZ-RNA结合构象中hZαADAR1的一些独特的结构特征。我们的结果表明,由hZαADAR1促进的RNA的AZ过渡表现出独特的结构和动态特征,可能与靶向ADAR1参与识别RNA底物有关。











































 京公网安备 11010802027423号
京公网安备 11010802027423号