当前位置:
X-MOL 学术
›
Mol. Pharmaceutics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Targeted Modification of the Cationic Anticancer Peptide HPRP-A1 with iRGD To Improve Specificity, Penetration, and Tumor-Tissue Accumulation
Molecular Pharmaceutics ( IF 4.5 ) Pub Date : 2018-12-28 00:00:00 , DOI: 10.1021/acs.molpharmaceut.8b00854 Cuihua Hu 1, 2 , Yibing Huang 1, 2 , Yuxin Chen 1, 2
Molecular Pharmaceutics ( IF 4.5 ) Pub Date : 2018-12-28 00:00:00 , DOI: 10.1021/acs.molpharmaceut.8b00854 Cuihua Hu 1, 2 , Yibing Huang 1, 2 , Yuxin Chen 1, 2
Affiliation
|
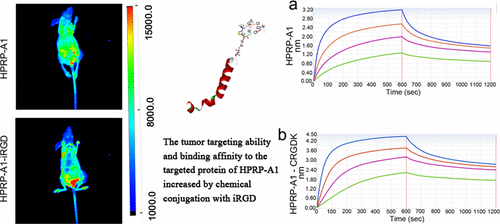
The chimeric peptide HPRP-A1-iRGD, composed of a chemically conjugated tumor-homing/penetration domain (iRGD) and a cationic anticancer peptide domain (HPRP-A1), was used to study the effect of targeted modification to enhance the peptide’s specificity, penetration, and tumor accumulation ability. The iRGD domain exhibits tumor-targeting and tumor-penetrating activities by specifically binding to the neuropilin-1 receptor. Acting as a homing/penetration domain, iRGD contributed to enhancing the tumor selectivity, permeability, and targeting of HPRP-A1 by targeted receptor dependence. As the anticancer active domain, HPRP-A1 kills cancer cells by disrupting the cell membrane and inducing apoptosis. The in vitro membrane selectivity toward cancer cells, such as A549 and MDA-MB-23, and human umbilical vein endothelial cells (HUVECs), normal cells, the penetrability assessment in the A549 3D multiple cell sphere model, and the in vivo tumor-tissue accumulation test in the A549 xenograft model indicated that HPRP-A1-iRGD exhibited significant increases in the selectivity toward membranes that highly express NRP-1, the penetration distance in 3D multiple cell spheres, and the accumulation in tumor tissues after intravenous injection, compared with HPRP-A1 alone. The mechanism of the enhanced targeting ability of HPRP-A1-iRGD was demonstrated by the pull-down assay and biolayer interferometry test, which indicated that the chimeric peptide could specifically bind to the neuropilin-1 protein with high affinity. We believe that chemical conjugation with iRGD to increase the specificity, penetration, and tumor-tissue accumulation of HPRP-A1 is an effective and promising approach for the targeted modification of peptides as anticancer therapeutics.
中文翻译:

靶向抗癌肽HPRP-A1的iRGD修饰,可提高特异性,穿透性和肿瘤组织积累。
嵌合肽HPRP-A1-iRGD由化学偶联的肿瘤归巢/穿透域(iRGD)和阳离子抗癌肽域(HPRP-A1)组成,用于研究靶向修饰以增强该肽的特异性的作用,穿透力和肿瘤蓄积能力。iRGD结构域通过特异性结合Neuropilin-1受体表现出靶向肿瘤和穿透肿瘤的活性。充当归巢/穿透域,iRGD有助于通过靶向受体依赖性增强肿瘤选择性,通透性和HPRP-A1靶向。作为抗癌活性域,HPRP-A1通过破坏细胞膜并诱导细胞凋亡来杀死癌细胞。在体外对A549和MDA-MB-23等癌细胞以及人脐静脉内皮细胞(HUVEC),正常细胞的膜选择性,在A549 3D多细胞球体模型中的渗透性评估以及体内在A549异种移植模型中进行的肿瘤组织蓄积测试表明,HPRP-A1-iRGD对高表达NRP-1的膜的选择性,在3D多个细胞球体中的穿透距离以及静脉注射后在肿瘤组织中的蓄积表现出显着提高,与仅HPRP-A1相比。通过下拉测定和生物层干涉试验证明了HPRP-A1-iRGD增强靶向能力的机制,表明该嵌合肽可以高亲和力特异性结合neuropilin-1蛋白。我们相信,与iRGD进行化学缀合以增加HPRP-A1的特异性,渗透性和肿瘤组织积聚是一种有效且有前途的方法,可用于靶向修饰多肽作为抗癌治疗剂。
更新日期:2018-12-28
中文翻译:

靶向抗癌肽HPRP-A1的iRGD修饰,可提高特异性,穿透性和肿瘤组织积累。
嵌合肽HPRP-A1-iRGD由化学偶联的肿瘤归巢/穿透域(iRGD)和阳离子抗癌肽域(HPRP-A1)组成,用于研究靶向修饰以增强该肽的特异性的作用,穿透力和肿瘤蓄积能力。iRGD结构域通过特异性结合Neuropilin-1受体表现出靶向肿瘤和穿透肿瘤的活性。充当归巢/穿透域,iRGD有助于通过靶向受体依赖性增强肿瘤选择性,通透性和HPRP-A1靶向。作为抗癌活性域,HPRP-A1通过破坏细胞膜并诱导细胞凋亡来杀死癌细胞。在体外对A549和MDA-MB-23等癌细胞以及人脐静脉内皮细胞(HUVEC),正常细胞的膜选择性,在A549 3D多细胞球体模型中的渗透性评估以及体内在A549异种移植模型中进行的肿瘤组织蓄积测试表明,HPRP-A1-iRGD对高表达NRP-1的膜的选择性,在3D多个细胞球体中的穿透距离以及静脉注射后在肿瘤组织中的蓄积表现出显着提高,与仅HPRP-A1相比。通过下拉测定和生物层干涉试验证明了HPRP-A1-iRGD增强靶向能力的机制,表明该嵌合肽可以高亲和力特异性结合neuropilin-1蛋白。我们相信,与iRGD进行化学缀合以增加HPRP-A1的特异性,渗透性和肿瘤组织积聚是一种有效且有前途的方法,可用于靶向修饰多肽作为抗癌治疗剂。











































 京公网安备 11010802027423号
京公网安备 11010802027423号