当前位置:
X-MOL 学术
›
Energy Environ. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Enhancing the activity of oxygen-evolution and chlorine-evolution electrocatalysts by atomic layer deposition of TiO2†
Energy & Environmental Science ( IF 32.4 ) Pub Date : 2018-12-14 00:00:00 , DOI: 10.1039/c8ee02351d Cody E. Finke 1, 2, 2, 3, 4 , Stefan T. Omelchenko 2, 3, 5 , Justin T. Jasper 1, 2, 2, 3, 4 , Michael F. Lichterman 1, 2, 3 , Carlos G. Read 2, 2, 3, 4, 6 , Nathan S. Lewis 2, 3, 6 , Michael R. Hoffmann 1, 2, 2, 3, 4
Energy & Environmental Science ( IF 32.4 ) Pub Date : 2018-12-14 00:00:00 , DOI: 10.1039/c8ee02351d Cody E. Finke 1, 2, 2, 3, 4 , Stefan T. Omelchenko 2, 3, 5 , Justin T. Jasper 1, 2, 2, 3, 4 , Michael F. Lichterman 1, 2, 3 , Carlos G. Read 2, 2, 3, 4, 6 , Nathan S. Lewis 2, 3, 6 , Michael R. Hoffmann 1, 2, 2, 3, 4
Affiliation
|
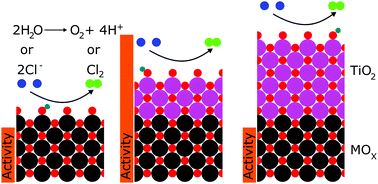
We report that TiO2 coatings formed via atomic layer deposition (ALD) may tune the activity of IrO2, RuO2, and FTO for the oxygen-evolution and chlorine-evolution reactions (OER and CER). Electrocatalysts exposed to ∼3–30 ALD cycles of TiO2 exhibited overpotentials at 10 mA cm−2 of geometric current density that were several hundred millivolts lower than uncoated catalysts, with correspondingly higher specific activities. For example, the deposition of TiO2 onto IrO2 yielded a 9-fold increase in the OER-specific activity in 1.0 M H2SO4 (0.1 to 0.9 mA cmECSA−2 at 350 mV overpotential). The oxidation state of titanium and the potential of zero charge were also a function of the number of ALD cycles, indicating a correlation between oxidation state, potential of zero charge, and activity of the tuned electrocatalysts.
中文翻译:

通过原子层沉积TiO 2 †增强析氧和析氯电催化剂的活性
我们报告说,通过原子层沉积(ALD)形成的TiO 2涂层可能会调节IrO 2,RuO 2和FTO的活性,以进行氧释放和氯释放反应(OER和CER)。暴露于TiO 2的约3–30 ALD循环的电催化剂在10 mA cm -2的几何电流密度下表现出的过电势比未涂覆的催化剂低几百毫伏,并且比活性更高。例如,将TiO 2沉积到IrO 2上,在1.0 MH 2 SO 4(0.1至0.9 mA cm ECSA中)的OER比活度增加了9倍。在350 mV超电势下为-2)。钛的氧化态和零电荷的电势也是ALD循环次数的函数,表明氧化态,零电荷的电势和调整后的电催化剂的活性之间存在相关性。
更新日期:2018-12-14
中文翻译:

通过原子层沉积TiO 2 †增强析氧和析氯电催化剂的活性
我们报告说,通过原子层沉积(ALD)形成的TiO 2涂层可能会调节IrO 2,RuO 2和FTO的活性,以进行氧释放和氯释放反应(OER和CER)。暴露于TiO 2的约3–30 ALD循环的电催化剂在10 mA cm -2的几何电流密度下表现出的过电势比未涂覆的催化剂低几百毫伏,并且比活性更高。例如,将TiO 2沉积到IrO 2上,在1.0 MH 2 SO 4(0.1至0.9 mA cm ECSA中)的OER比活度增加了9倍。在350 mV超电势下为-2)。钛的氧化态和零电荷的电势也是ALD循环次数的函数,表明氧化态,零电荷的电势和调整后的电催化剂的活性之间存在相关性。










































 京公网安备 11010802027423号
京公网安备 11010802027423号