Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Acetylcholinesterase Choline-Based Ionic Liquid Inhibitors: In Vitro and in Silico Molecular Docking Studies
ACS Omega ( IF 3.7 ) Pub Date : 2018-12-12 00:00:00 , DOI: 10.1021/acsomega.8b02347 Filipa Siopa 1 , Raquel F. M. Frade 1 , Ana Diniz 2 , Joana M. Andrade 3 , Marisa Nicolai 3 , Ana Meirinhos 1 , Susana D. Lucas 1 , Filipa Marcelo 2 , Carlos A. M. Afonso 1 , Patrícia Rijo 1, 3
ACS Omega ( IF 3.7 ) Pub Date : 2018-12-12 00:00:00 , DOI: 10.1021/acsomega.8b02347 Filipa Siopa 1 , Raquel F. M. Frade 1 , Ana Diniz 2 , Joana M. Andrade 3 , Marisa Nicolai 3 , Ana Meirinhos 1 , Susana D. Lucas 1 , Filipa Marcelo 2 , Carlos A. M. Afonso 1 , Patrícia Rijo 1, 3
Affiliation
|
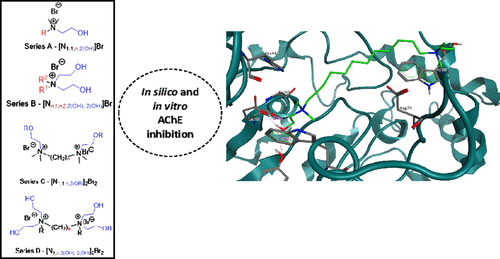
Monocationic and dicationic cholinium ionic liquids (ILs) were synthesized and evaluated as acetylcholinesterase (AChE) inhibitors with in vitro and in silico models, and their cytotoxicity was assessed using human cell lines from skin (CRL-1502) and colon cancer (CaCo-2). The ILs with a longer alkyl chain were stronger AChE inhibitors, the dicationic ILs (DILs) being more active than the monocationic ILs. The best result was obtained for [N1,1,12,2(OH)]2Br2 at a concentration of 0.18 μM by reducing half enzyme activity without affecting the viability of tested cell lines. A saturation-transfer difference NMR (STD-NMR) binding study was carried out, demonstrating that [N1,1,12,2(OH)]2Br2 binds to AChE. STD-NMR competition binding experiments, using galanthamine as a reference ligand, clearly highlight that the IL displaces galanthamine in the AChE binding site pinpointing [N1,1,12,2(OH)]2Br2 inside the deep gorge of AChE. In order to obtain a three-dimensional (3D) view of the molecular recognition process, in silico molecular docking studies on the active site of AChE were carried out. The proposed 3D model of the AChE/DIL complex is in agreement with the STD-derived epitope mapping, which explains the competition with galanthamine and unveils key interactions in both peripheral and catalytic sites of AChE. These interactions seem essential to govern the recognition of DILs by the AChE enzyme. Our study provides a structural and functional platform that can be used for the rational design of choline-based ILs as potent AChE inhibitors.
中文翻译:

基于乙酰胆碱酯酶胆碱的离子液体抑制剂:体外和计算机模拟分子对接研究
合成了单阳离子和离子型胆碱离子液体(ILs),并作为乙酰胆碱酯酶(AChE)抑制剂在体外和计算机模拟中进行了评估,并使用来自皮肤的人类细胞系(CRL-1502)和结肠癌(CaCo-2)评估了它们的细胞毒性)。具有更长烷基链的IL是更强的AChE抑制剂,其离子型IL(DIL)比单阳离子型ILs具有更高的活性。对于[N 1,1,12,2(OH) ] 2 Br 2,在不影响被测细胞系活力的情况下,通过降低其一半酶活性,可获得0.18μM浓度的最佳结果。进行了饱和转移差异NMR(STD-NMR)结合研究,表明[N 1,1,12,2(OH) ] 2 Br 2绑定到AChE。使用加兰他敏作为参考配体的STD-NMR竞争结合实验清楚地表明,IL取代了AChE结合位点中的加兰他敏,精确定位了[N 1,1,12,2(OH) ] 2 Br 2在AChE的深深峡谷中。为了获得分子识别过程的三维(3D)视图,对AChE活性位点进行了计算机分子对接研究。拟议的AChE / DIL复合物的3D模型与STD衍生的表位作图一致,这解释了与加兰他敏的竞争,并揭示了AChE外围和催化位点的关键相互作用。这些相互作用似乎对于控制AChE酶识别DIL至关重要。我们的研究提供了一个结构和功能平台,可用于合理设计胆碱基ILs作为有效的AChE抑制剂。
更新日期:2018-12-12
中文翻译:

基于乙酰胆碱酯酶胆碱的离子液体抑制剂:体外和计算机模拟分子对接研究
合成了单阳离子和离子型胆碱离子液体(ILs),并作为乙酰胆碱酯酶(AChE)抑制剂在体外和计算机模拟中进行了评估,并使用来自皮肤的人类细胞系(CRL-1502)和结肠癌(CaCo-2)评估了它们的细胞毒性)。具有更长烷基链的IL是更强的AChE抑制剂,其离子型IL(DIL)比单阳离子型ILs具有更高的活性。对于[N 1,1,12,2(OH) ] 2 Br 2,在不影响被测细胞系活力的情况下,通过降低其一半酶活性,可获得0.18μM浓度的最佳结果。进行了饱和转移差异NMR(STD-NMR)结合研究,表明[N 1,1,12,2(OH) ] 2 Br 2绑定到AChE。使用加兰他敏作为参考配体的STD-NMR竞争结合实验清楚地表明,IL取代了AChE结合位点中的加兰他敏,精确定位了[N 1,1,12,2(OH) ] 2 Br 2在AChE的深深峡谷中。为了获得分子识别过程的三维(3D)视图,对AChE活性位点进行了计算机分子对接研究。拟议的AChE / DIL复合物的3D模型与STD衍生的表位作图一致,这解释了与加兰他敏的竞争,并揭示了AChE外围和催化位点的关键相互作用。这些相互作用似乎对于控制AChE酶识别DIL至关重要。我们的研究提供了一个结构和功能平台,可用于合理设计胆碱基ILs作为有效的AChE抑制剂。











































 京公网安备 11010802027423号
京公网安备 11010802027423号