Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Phase Transformation and Thermal Decomposition Kinetics of a Mixed Rare Earth Concentrate
ACS Omega ( IF 4.1 ) Pub Date : 2018-12-11 00:00:00 , DOI: 10.1021/acsomega.8b01140 Dan Zou 1 , Ji Chen 1 , Kai Li 1, 2 , Deqian Li 1
ACS Omega ( IF 4.1 ) Pub Date : 2018-12-11 00:00:00 , DOI: 10.1021/acsomega.8b01140 Dan Zou 1 , Ji Chen 1 , Kai Li 1, 2 , Deqian Li 1
Affiliation
|
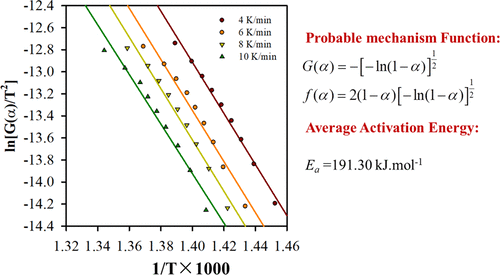
The Bayan Obo rare earth (BORE) concentrate is the largest light rare earth mineral resource in the world. The decomposition of the BORE concentrate is a principal step to develop new technologies to recover useful elements and is therefore of much importance. In this paper, the phase transformation and thermal decomposition kinetics of the BORE concentrate in air were investigated. The results showed that bastnaesite decomposed upon air roasting. The roasted solid was recovered by sulfuric acid leaching. The activation energy and pre-exponential factor of the roasting reaction of the BORE concentrate were calculated through the isoconversional methods of Flynn–Wall–Ozawa and Kissinger, and the probable mechanism function was estimated through the Coats–Redfern method. The kinetic model that best describes the thermal decomposition reaction for the BORE concentrate is the A2 (Avrami–Erofeev 2) model, and the corresponding probable mechanism function is f(α) = 2(1 – α)[−ln(1 – α)]1/2 and G(α) = [−ln(1 – α)]1/2.
中文翻译:

混合稀土精矿的相变和热分解动力学
巴彦鄂博稀土精矿是世界上最大的轻稀土矿产资源。BORE精矿的分解是开发新技术以回收有用元素的主要步骤,因此非常重要。本文研究了空气中BORE精矿的相变和热分解动力学。结果表明,焙烧时贝氏体分解。焙烧的固体通过硫酸浸提回收。通过Flynn-Wall-Ozawa和Kissinger的等转换方法计算了BORE精矿焙烧反应的活化能和指数前因子,并通过Coats-Redfern方法估算了可能的机理函数。f(α)= 2(1 –α)[-ln(1 –α)] 1/2和G(α)= [−ln(1 –α)] 1/2。
更新日期:2018-12-11
中文翻译:

混合稀土精矿的相变和热分解动力学
巴彦鄂博稀土精矿是世界上最大的轻稀土矿产资源。BORE精矿的分解是开发新技术以回收有用元素的主要步骤,因此非常重要。本文研究了空气中BORE精矿的相变和热分解动力学。结果表明,焙烧时贝氏体分解。焙烧的固体通过硫酸浸提回收。通过Flynn-Wall-Ozawa和Kissinger的等转换方法计算了BORE精矿焙烧反应的活化能和指数前因子,并通过Coats-Redfern方法估算了可能的机理函数。f(α)= 2(1 –α)[-ln(1 –α)] 1/2和G(α)= [−ln(1 –α)] 1/2。



























 京公网安备 11010802027423号
京公网安备 11010802027423号