Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Physicochemical and Ion-Sensing Properties of Benzofurazan-Appended Calix[4]arene in Solution and on Gold Nanoparticles: Spectroscopy, Microscopy, and DFT Computations in Support of the Species of Recognition
ACS Omega ( IF 3.7 ) Pub Date : 2018-12-11 00:00:00 , DOI: 10.1021/acsomega.8b02848 Bhawna Uttam 1 , M. Althaf Hussain 1 , Sunita Joshi 1 , Chebrolu Pulla Rao 1
ACS Omega ( IF 3.7 ) Pub Date : 2018-12-11 00:00:00 , DOI: 10.1021/acsomega.8b02848 Bhawna Uttam 1 , M. Althaf Hussain 1 , Sunita Joshi 1 , Chebrolu Pulla Rao 1
Affiliation
|
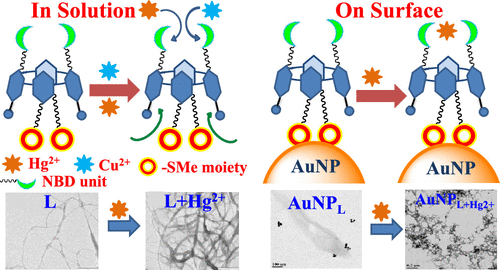
A calix[4]arene conjugate (L) functionalized at the lower rim with a benzofurazan fluorophore (NBD) and at the upper rim with a thioether moiety has been synthesized and characterized by 1H NMR, 13C NMR, and mass spectrometry techniques. Both the absorption and emission spectral data for L in different solvents exhibited progressive changes with an increase in polarity. Ion recognition studies were performed by absorption and fluorescence spectroscopy using 10 different metal ions. Among these, Hg2+ exhibited greater changes in these spectra, whereas Cu2+ showed only significant changes and all other ions showed no change in the spectral features. Although the Hg2+ has dominant influence on the spectral features and provides a detection limit of 56.0 ± 0.6 ppb, the selectivity was hampered because of the presence of the derivatizations present on both the rims of L for ion interaction in solution. Therefore, L was immobilized onto gold nanoparticles (AuNPL’s) so that the upper rim derivatizations anchor onto the gold surface through Au–S interactions, and this leaves out only the lower rim NBD derivatization for interaction with ions selectively. The AuNPL’s were characterized by transmission electron microscopy, scanning electron microscopy, energy-dispersive X-ray spectroscopy, and X-ray photoelectron spectroscopy (XPS) analyses. The surface characteristics were analyzed by contact angle measurements. The AuNPL’s exhibit greater selectivity and enhanced sensitivity for Hg2+ ions with a lowest detection limit of 48.0 ± 0.8 ppb. The immobilization of L onto AuNPs was reflected in the corresponding fluorescence lifetime values, and the addition of Hg2+ to either L or AuNPL showed fluorescence quenching. The reversible recognition of Hg2+ by L was demonstrated by titrating L or AuNPL with Hg2+ followed by tetra-butyl ammonium iodide for several cycles. The structural features of Hg2+-bound species were demonstrated by density functional theory computations and were supported by the XPS data. The Hg2+ induces aggregated fibrillar morphology into supramolecular L, as demonstrated by microscopy when Hg2+ was added either to L or to AuNPL, supporting aggregation-caused quenching.
中文翻译:

溶液中和金纳米粒子上附有苯并呋喃山的杯[4]芳烃的理化和离子传感特性:光谱,显微镜和DFT计算以支持识别物种
已合成并通过1 H NMR,13 C NMR和质谱技术对在下部边缘被苯并呋喃山荧光团(NBD)和在上部边缘被硫醚部分官能化的杯[4]芳烃共轭物(L)进行了表征。L在不同溶剂中的吸收光谱和发射光谱数据都显示出随着极性的增加而逐渐变化的趋势。离子识别研究是使用10种不同的金属离子通过吸收光谱和荧光光谱进行的。其中,Hg 2+在这些光谱中显示出较大的变化,而Cu 2+仅显示出显着变化,而所有其他离子均未显示出光谱特征的变化。虽然汞2+对光谱特征具有主要影响,检测极限为56.0±0.6 ppb,由于溶液L的两个边缘上都存在衍生化作用,离子之间的相互作用,选择性受到阻碍。因此,将L固定在金纳米颗粒(AuNP L)上,以便上边缘的衍生化通过Au-S相互作用锚定在金表面上,而只剩下下边缘NBD衍生化以选择性地与离子相互作用。该金纳米粒子大号通过透射电子显微镜,扫描电子显微镜,能量色散X射线光谱学和X射线光电子光谱学(XPS)分析来表征。通过接触角测量来分析表面特性。AuNP L对Hg 2+离子具有更高的选择性和更高的灵敏度,最低检测限为48.0±0.8 ppb。L固定在AuNPs上反映在相应的荧光寿命值中,向L或AuNP L中添加Hg 2+均显示出荧光猝灭。汞柱的可逆识别2+由大号通过滴定证实L或具有Hg 2+的AuNP L,然后加入四丁基碘化铵进行数个循环。Hg 2+结合物种的结构特征通过密度泛函理论计算得到证明,并得到XPS数据的支持。Hg 2+将聚集的纤维状形态诱导成超分子L,如在向L或AuNP L中添加Hg 2+的显微镜观察所证明,支持聚集引起的猝灭。
更新日期:2018-12-11
中文翻译:

溶液中和金纳米粒子上附有苯并呋喃山的杯[4]芳烃的理化和离子传感特性:光谱,显微镜和DFT计算以支持识别物种
已合成并通过1 H NMR,13 C NMR和质谱技术对在下部边缘被苯并呋喃山荧光团(NBD)和在上部边缘被硫醚部分官能化的杯[4]芳烃共轭物(L)进行了表征。L在不同溶剂中的吸收光谱和发射光谱数据都显示出随着极性的增加而逐渐变化的趋势。离子识别研究是使用10种不同的金属离子通过吸收光谱和荧光光谱进行的。其中,Hg 2+在这些光谱中显示出较大的变化,而Cu 2+仅显示出显着变化,而所有其他离子均未显示出光谱特征的变化。虽然汞2+对光谱特征具有主要影响,检测极限为56.0±0.6 ppb,由于溶液L的两个边缘上都存在衍生化作用,离子之间的相互作用,选择性受到阻碍。因此,将L固定在金纳米颗粒(AuNP L)上,以便上边缘的衍生化通过Au-S相互作用锚定在金表面上,而只剩下下边缘NBD衍生化以选择性地与离子相互作用。该金纳米粒子大号通过透射电子显微镜,扫描电子显微镜,能量色散X射线光谱学和X射线光电子光谱学(XPS)分析来表征。通过接触角测量来分析表面特性。AuNP L对Hg 2+离子具有更高的选择性和更高的灵敏度,最低检测限为48.0±0.8 ppb。L固定在AuNPs上反映在相应的荧光寿命值中,向L或AuNP L中添加Hg 2+均显示出荧光猝灭。汞柱的可逆识别2+由大号通过滴定证实L或具有Hg 2+的AuNP L,然后加入四丁基碘化铵进行数个循环。Hg 2+结合物种的结构特征通过密度泛函理论计算得到证明,并得到XPS数据的支持。Hg 2+将聚集的纤维状形态诱导成超分子L,如在向L或AuNP L中添加Hg 2+的显微镜观察所证明,支持聚集引起的猝灭。











































 京公网安备 11010802027423号
京公网安备 11010802027423号