Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Interaction of CO and CH4 Adsorption with Noble Metal (Rh, Pd, and Pt)-Decorated N3-CNTs: A First-Principles Study
ACS Omega ( IF 3.7 ) Pub Date : 2018-12-07 00:00:00 , DOI: 10.1021/acsomega.8b02578 Hao Cui 1, 2 , Xiaoxing Zhang 1, 3 , Jun Zhang 1 , Muhammad Ali Mehmood 1
ACS Omega ( IF 3.7 ) Pub Date : 2018-12-07 00:00:00 , DOI: 10.1021/acsomega.8b02578 Hao Cui 1, 2 , Xiaoxing Zhang 1, 3 , Jun Zhang 1 , Muhammad Ali Mehmood 1
Affiliation
|
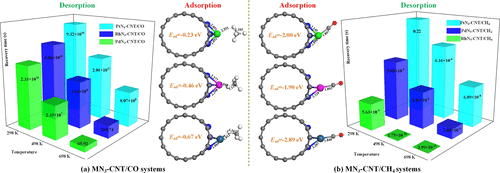
Using a first-principles theory, the structural and electronic behaviors of noble metal atom (Rh, Pd, and Pt)-decorated N3-CNTs were investigated. Meanwhile, the adsorption behavior of CO and CH4 molecules onto Rh-, Pd-, and Pt-decorated N3-CNTs was studied as well to exploit their potential applications. Results indicate that noble metal atoms are likely to be adsorbed on the N3 center of pyridine-like N3-CNTs under electrophilic attack. Moreover, the noble metal-embedded N3 group would provide CNTs with enhanced performance for gas adsorption compared with noble metal-embedded surfaces because of the improvement of chemical activity and electron mobility in our proposed configurations. The findings in this report would be beneficial for exploiting a possible adsorbent for CO scavenging with excellent adsorbing ability and a possible sensor for CH4 detection with good sensitivity and recovery behavior.
中文翻译:

CO 和 CH4 吸附与贵金属(Rh、Pd 和 Pt)修饰的 N3-CNT 的相互作用:第一性原理研究
利用第一性原理理论,研究了贵金属原子(Rh、Pd和Pt)修饰的N 3 -CNT的结构和电子行为。同时,还研究了CO和CH 4分子在Rh、Pd和Pt修饰的N 3 -CNT上的吸附行为,以开发其潜在的应用。结果表明,在亲电攻击下,贵金属原子很可能吸附在类吡啶N 3 -CNT的N 3中心上。此外,由于我们提出的结构中化学活性和电子迁移率的提高,与贵金属嵌入的表面相比,贵金属嵌入的N 3基团将为CNT提供增强的气体吸附性能。本报告中的研究结果将有利于开发一种可能的具有优异吸附能力的 CO 清除吸附剂,以及一种具有良好灵敏度和恢复性能的用于 CH 4检测的传感器。
更新日期:2018-12-07
中文翻译:

CO 和 CH4 吸附与贵金属(Rh、Pd 和 Pt)修饰的 N3-CNT 的相互作用:第一性原理研究
利用第一性原理理论,研究了贵金属原子(Rh、Pd和Pt)修饰的N 3 -CNT的结构和电子行为。同时,还研究了CO和CH 4分子在Rh、Pd和Pt修饰的N 3 -CNT上的吸附行为,以开发其潜在的应用。结果表明,在亲电攻击下,贵金属原子很可能吸附在类吡啶N 3 -CNT的N 3中心上。此外,由于我们提出的结构中化学活性和电子迁移率的提高,与贵金属嵌入的表面相比,贵金属嵌入的N 3基团将为CNT提供增强的气体吸附性能。本报告中的研究结果将有利于开发一种可能的具有优异吸附能力的 CO 清除吸附剂,以及一种具有良好灵敏度和恢复性能的用于 CH 4检测的传感器。











































 京公网安备 11010802027423号
京公网安备 11010802027423号