Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis and Characterization of N,N′-Bismesityl Phenanthrene-9,10-diimine and Imine–Nitrone
ACS Omega ( IF 3.7 ) Pub Date : 2018-12-07 00:00:00 , DOI: 10.1021/acsomega.8b02981 Chao Dong 1 , Diane A Dickie 2 , William A Maio 1 , Thomas A Manz 1
ACS Omega ( IF 3.7 ) Pub Date : 2018-12-07 00:00:00 , DOI: 10.1021/acsomega.8b02981 Chao Dong 1 , Diane A Dickie 2 , William A Maio 1 , Thomas A Manz 1
Affiliation
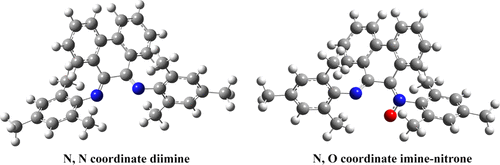
|
The sterically bulky compounds N,N′-bismesityl phenanthrene-9,10-diimine [1] and imine–nitrone [2] were synthesized. To the best of our knowledge, this is the first report of the synthesis of a bulky steric imine–nitrone accessed from the secondary ketimine using urea hydrogen peroxide over methyltrioxorhenium catalyst. Purified compounds were characterized using 1H and 13C NMR, high-resolution mass spectrometry, and infrared spectrometry. We report the first crystal structure of compound 1. Detailed IR bands of compounds 1 and 2 were assigned by comparing experimentally measured spectra to individually animated modes of quantum mechanically computed spectra. We believe these compounds may be of use as bidentate ligands in the synthesis of novel organometallic compounds. The asymmetric N and O coordination sites of compound 2 might impart interesting electronic effects to organometallic compounds compared to the symmetric N,N′-coordination sites of compound 1.
中文翻译:

N,N′-双三苯基菲-9,10-二亚胺和亚胺硝酮的合成与表征
合成了空间大的化合物N , N'-双三苯基菲-9,10-二亚胺[ 1 ]和亚胺-硝酮[ 2 ]。据我们所知,这是第一份关于在甲基三氧铼催化剂上使用过氧化氢尿素从二级酮亚胺合成大体积立体亚胺硝酮的报告。使用1 H 和13 C NMR、高分辨率质谱和红外光谱对纯化的化合物进行了表征。我们报告了化合物1的第一个晶体结构。通过将实验测量的光谱与量子力学计算光谱的单独动画模式进行比较,分配了化合物1和2的详细红外波段。我们相信这些化合物可用作新型有机金属化合物合成中的二齿配体。与化合物1的对称 N 、 N'-配位位点相比,化合物2的不对称N和O配位位点可能会给有机金属化合物带来有趣的电子效应。
更新日期:2018-12-07
中文翻译:

N,N′-双三苯基菲-9,10-二亚胺和亚胺硝酮的合成与表征
合成了空间大的化合物N , N'-双三苯基菲-9,10-二亚胺[ 1 ]和亚胺-硝酮[ 2 ]。据我们所知,这是第一份关于在甲基三氧铼催化剂上使用过氧化氢尿素从二级酮亚胺合成大体积立体亚胺硝酮的报告。使用1 H 和13 C NMR、高分辨率质谱和红外光谱对纯化的化合物进行了表征。我们报告了化合物1的第一个晶体结构。通过将实验测量的光谱与量子力学计算光谱的单独动画模式进行比较,分配了化合物1和2的详细红外波段。我们相信这些化合物可用作新型有机金属化合物合成中的二齿配体。与化合物1的对称 N 、 N'-配位位点相比,化合物2的不对称N和O配位位点可能会给有机金属化合物带来有趣的电子效应。










































 京公网安备 11010802027423号
京公网安备 11010802027423号