当前位置:
X-MOL 学术
›
Chem. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Chiral iminophosphorane catalyzed asymmetric sulfenylation of 4-substituted pyrazolones†
Chemical Communications ( IF 4.3 ) Pub Date : 2018-12-06 00:00:00 , DOI: 10.1039/c8cc09049a Jianwei Han 1, 2, 3, 4, 5 , Yanxia Zhang 1, 2, 3, 4, 5 , Xin-Yan Wu 1, 2, 3, 4, 5 , Henry N. C. Wong 6, 7, 8, 9, 10
Chemical Communications ( IF 4.3 ) Pub Date : 2018-12-06 00:00:00 , DOI: 10.1039/c8cc09049a Jianwei Han 1, 2, 3, 4, 5 , Yanxia Zhang 1, 2, 3, 4, 5 , Xin-Yan Wu 1, 2, 3, 4, 5 , Henry N. C. Wong 6, 7, 8, 9, 10
Affiliation
|
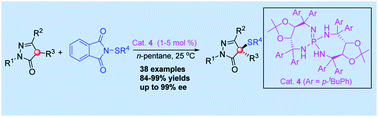
An excellent level of enantioselectivity in asymmetric sulfenylation of 4-substituted pyrazolones was achieved with chiral iminophosphorane as the organocatalyst under continuum solvation conditions (up to 99% ee). Importantly, this catalytic process features high efficiency with excellent enantioselectivities, easy separation of products, low catalytic loadings and scale-up to grams without loss of enantioselectivity.
中文翻译:

手性亚氨基膦烷催化4-取代的吡唑啉酮的不对称亚磺酰基†
在连续溶剂化条件下(手感高达99%ee),使用手性亚氨基膦烷作为有机催化剂,可以实现4-取代吡唑啉酮不对称亚磺酰化反应中优异的对映选择性。重要的是,该催化过程的特点是效率高,对映选择性优异,产物易于分离,催化负载低,按比例放大至克,而不会损失对映选择性。
更新日期:2018-12-06
中文翻译:

手性亚氨基膦烷催化4-取代的吡唑啉酮的不对称亚磺酰基†
在连续溶剂化条件下(手感高达99%ee),使用手性亚氨基膦烷作为有机催化剂,可以实现4-取代吡唑啉酮不对称亚磺酰化反应中优异的对映选择性。重要的是,该催化过程的特点是效率高,对映选择性优异,产物易于分离,催化负载低,按比例放大至克,而不会损失对映选择性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号