当前位置:
X-MOL 学术
›
Mol. Pharmaceutics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Insight into Amorphous Solid Dispersion Performance by Coupled Dissolution and Membrane Mass Transfer Measurements
Molecular Pharmaceutics ( IF 4.5 ) Pub Date : 2018-12-06 00:00:00 , DOI: 10.1021/acs.molpharmaceut.8b01117 Siddhi S. Hate 1 , Susan M. Reutzel-Edens 2 , Lynne S. Taylor 1
Molecular Pharmaceutics ( IF 4.5 ) Pub Date : 2018-12-06 00:00:00 , DOI: 10.1021/acs.molpharmaceut.8b01117 Siddhi S. Hate 1 , Susan M. Reutzel-Edens 2 , Lynne S. Taylor 1
Affiliation
|
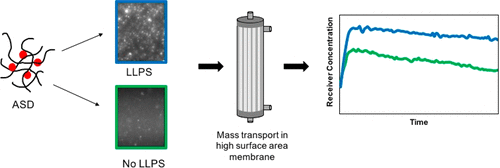
The tendency of highly supersaturated solutions of poorly water-soluble drugs to undergo liquid–liquid phase separation (LLPS) into drug-rich and water-rich phases when the concentration exceeds the amorphous solubility, for example, during dissolution of some amorphous solid dispersions, is thought to be advantageous from a bioavailability enhancement perspective. Recently, we have developed a high surface area, flow-through absorptive dissolution testing apparatus that enables fast mass transfer providing more in vivo relevant conditions and time frames for formulation testing. Using this apparatus, the absorption behaviors of solutions with different extents of supersaturation below and above the amorphous solubility were evaluated. In addition, simultaneous dissolution–absorption testing of amorphous solid dispersions (ASDs) with varying drug loadings and polymer types was carried out to study and distinguish the absorption behavior of ASDs that do or do not undergo LLPS. When compared with closed-compartment dissolution testing, a significant influence of the absorptive compartment on the dissolution rate of ASDs, particularly at high drug loadings, was observed. The formation of drug-rich nanodroplets, generated by both solvent-addition and ASD dissolution, resulted in a higher amount of drug transferred across the membrane. Moreover, the mass transfer was further enhanced with increasing concentration above the amorphous solubility, thereby showing correlation with an increase in the number of drug-rich particles. The importance of including an absorptive compartment in dissolution testing is highlighted in this study, enabling coupling of dissolution to membrane transport, and providing a more meaningful comparison between different formulations.
中文翻译:

通过耦合溶解和膜传质测量了解无定形固体分散性能
当浓度超过无定形溶解度时,例如在某些无定形固体分散体的溶解过程中,水溶性差的药物的高度过饱和溶液会经历液相-液相分离(LLPS)成为富药相和富水相的趋势,从提高生物利用度的角度来看,“大豆”被认为是有利的。最近,我们开发了一种高表面积,流通式吸收溶出度测试设备,该设备可实现快速传质,从而为制剂测试提供更多的体内相关条件和时间表。使用该设备,评估了在非晶态溶解度以下和之上具有不同过饱和程度的溶液的吸收行为。此外,同时进行了具有不同载药量和聚合物类型的无定形固体分散体(ASD)的溶出-吸收试验,以研究和区分进行或不进行LLPS的ASD的吸收行为。与封闭室溶出度测试相比,吸收区对ASD溶出度有显着影响,尤其是在高载药量下。通过溶剂添加和ASD溶解产生的药物富集的纳米液滴的形成导致跨膜转移的药物量更高。此外,随着无定形溶解度以上浓度的增加,传质进一步增强,从而显示出与富含药物的颗粒数目增加的相关性。
更新日期:2018-12-06
中文翻译:

通过耦合溶解和膜传质测量了解无定形固体分散性能
当浓度超过无定形溶解度时,例如在某些无定形固体分散体的溶解过程中,水溶性差的药物的高度过饱和溶液会经历液相-液相分离(LLPS)成为富药相和富水相的趋势,从提高生物利用度的角度来看,“大豆”被认为是有利的。最近,我们开发了一种高表面积,流通式吸收溶出度测试设备,该设备可实现快速传质,从而为制剂测试提供更多的体内相关条件和时间表。使用该设备,评估了在非晶态溶解度以下和之上具有不同过饱和程度的溶液的吸收行为。此外,同时进行了具有不同载药量和聚合物类型的无定形固体分散体(ASD)的溶出-吸收试验,以研究和区分进行或不进行LLPS的ASD的吸收行为。与封闭室溶出度测试相比,吸收区对ASD溶出度有显着影响,尤其是在高载药量下。通过溶剂添加和ASD溶解产生的药物富集的纳米液滴的形成导致跨膜转移的药物量更高。此外,随着无定形溶解度以上浓度的增加,传质进一步增强,从而显示出与富含药物的颗粒数目增加的相关性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号