当前位置:
X-MOL 学术
›
Integr. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Enhanced intrinsic CYP3A4 activity in human hepatic C3A cells with optically controlled CRISPR/dCas9 activator complex
Integrative Biology ( IF 1.5 ) Pub Date : 2018-12-06 , DOI: 10.1039/c8ib00109j Shuo Han 1, 2, 3, 4, 5 , Shiruo Wei 1, 2, 3, 4 , Xuan Wang 6, 7, 8, 9, 10 , Xu Han 6, 7, 8, 9, 10 , Mingzhi Zhang 1, 2, 3, 4 , Ming Su 1, 2, 3, 4 , Yang Li 6, 7, 8, 9, 10 , Jinhai Guo 3, 4, 11 , Wotan Zeng 3, 4, 11 , Jinwen Liu 3, 4, 11 , Yi Gao 6, 7, 8, 9, 10 , Li Shen 1, 2, 3, 4
Integrative Biology ( IF 1.5 ) Pub Date : 2018-12-06 , DOI: 10.1039/c8ib00109j Shuo Han 1, 2, 3, 4, 5 , Shiruo Wei 1, 2, 3, 4 , Xuan Wang 6, 7, 8, 9, 10 , Xu Han 6, 7, 8, 9, 10 , Mingzhi Zhang 1, 2, 3, 4 , Ming Su 1, 2, 3, 4 , Yang Li 6, 7, 8, 9, 10 , Jinhai Guo 3, 4, 11 , Wotan Zeng 3, 4, 11 , Jinwen Liu 3, 4, 11 , Yi Gao 6, 7, 8, 9, 10 , Li Shen 1, 2, 3, 4
Affiliation
|
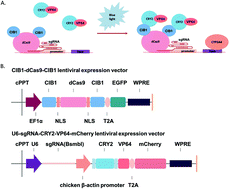
Human hepatic C3A cells have been applied in bioartificial liver development, although these cells display low intrinsic cytochrome P450 3A4 (CYP3A4) enzyme activity. We aimed to enhance CYP3A4 enzyme activity of C3A cells utilizing CRISPR gene editing technology. We designed two CYP3A4 expression enhanced systems applying clustered regularly interspaced short palindromic repeats (CRISPR) gene technology: a CRISPR-on activation system including dCas9-VP64-GFP and two U6-sgRNA-mCherry elements, and a light-controlled CRISPR-on activation system combining our CRISPR-on activation system with an optical control system to facilitate regulation of CYP3A4 expression for various applications. Results of enzymatic activity assays displayed increased CYP3A4 activity in C3A cells expressing the CRISPR-on activation system compared with C3A cells. In addition, CYP3A4 activity increased in C3A cells expressing the light-controlled CRISPR-on activation system under blue light radiation compared with C3A cells. Notably, there was no statistical difference in the increase of CYP3A4 protein amounts induced by these two methods. After expansion in culture, C3A cells with the light-controlled CRISPR-on activation system exhibited no statistical difference in CYP3A4 mRNA levels between generations. Our findings provide a method to stably enhance functional gene expression in bioartificial liver cells with the potential for large-scale cell expansion.
中文翻译:

光学控制的CRISPR / dCas9激活物复合物增强人肝C3A细胞的固有CYP3A4活性
人类肝C3A细胞已被应用于生物人工肝的发展,尽管这些细胞显示出较低的固有细胞色素P450 3A4(CYP3A4)酶活性。我们旨在利用CRISPR基因编辑技术来增强C3A细胞的CYP3A4酶活性。我们设计了两个CYP3A4表达增强系统,这些系统应用聚类的规则间隔的短回文重复(CRISPR)基因技术:一个CRISPR-on激活系统,包括dCas9-VP64-GFP和两个U6-sgRNA-mCherry元件,以及一个光控CRISPR-on激活系统结合了我们的CRISPR-on激活系统和光学控制系统,可促进针对各种应用的CYP3A4表达的调节。酶活性测定的结果显示,与C3A细胞相比,表达CRISPR-on激活系统的C3A细胞中CYP3A4活性增加。此外,与C3A细胞相比,在蓝光辐射下表达光控CRISPR-on激活系统的C3A细胞中CYP3A4活性增加。值得注意的是,由这两种方法诱导的CYP3A4蛋白量的增加没有统计学差异。在培养物中扩增后,具有光控CRISPR-on激活系统的C3A细胞在各代之间的CYP3A4 mRNA水平上无统计学差异。我们的发现提供了一种方法,可以稳定地增强生物人工肝细胞中的功能基因表达,并具有大规模细胞扩增的潜力。这两种方法诱导的CYP3A4蛋白量的增加没有统计学差异。在培养物中扩增后,具有光控CRISPR-on激活系统的C3A细胞在各代之间的CYP3A4 mRNA水平上无统计学差异。我们的发现提供了一种方法,可以稳定地增强生物人工肝细胞中的功能基因表达,并具有大规模细胞扩增的潜力。这两种方法诱导的CYP3A4蛋白量的增加没有统计学差异。在培养物中扩增后,具有光控CRISPR-on激活系统的C3A细胞在各代之间的CYP3A4 mRNA水平上无统计学差异。我们的发现提供了一种方法,可以稳定地增强生物人工肝细胞中的功能基因表达,并具有大规模细胞扩增的潜力。
更新日期:2018-12-06
中文翻译:

光学控制的CRISPR / dCas9激活物复合物增强人肝C3A细胞的固有CYP3A4活性
人类肝C3A细胞已被应用于生物人工肝的发展,尽管这些细胞显示出较低的固有细胞色素P450 3A4(CYP3A4)酶活性。我们旨在利用CRISPR基因编辑技术来增强C3A细胞的CYP3A4酶活性。我们设计了两个CYP3A4表达增强系统,这些系统应用聚类的规则间隔的短回文重复(CRISPR)基因技术:一个CRISPR-on激活系统,包括dCas9-VP64-GFP和两个U6-sgRNA-mCherry元件,以及一个光控CRISPR-on激活系统结合了我们的CRISPR-on激活系统和光学控制系统,可促进针对各种应用的CYP3A4表达的调节。酶活性测定的结果显示,与C3A细胞相比,表达CRISPR-on激活系统的C3A细胞中CYP3A4活性增加。此外,与C3A细胞相比,在蓝光辐射下表达光控CRISPR-on激活系统的C3A细胞中CYP3A4活性增加。值得注意的是,由这两种方法诱导的CYP3A4蛋白量的增加没有统计学差异。在培养物中扩增后,具有光控CRISPR-on激活系统的C3A细胞在各代之间的CYP3A4 mRNA水平上无统计学差异。我们的发现提供了一种方法,可以稳定地增强生物人工肝细胞中的功能基因表达,并具有大规模细胞扩增的潜力。这两种方法诱导的CYP3A4蛋白量的增加没有统计学差异。在培养物中扩增后,具有光控CRISPR-on激活系统的C3A细胞在各代之间的CYP3A4 mRNA水平上无统计学差异。我们的发现提供了一种方法,可以稳定地增强生物人工肝细胞中的功能基因表达,并具有大规模细胞扩增的潜力。这两种方法诱导的CYP3A4蛋白量的增加没有统计学差异。在培养物中扩增后,具有光控CRISPR-on激活系统的C3A细胞在各代之间的CYP3A4 mRNA水平上无统计学差异。我们的发现提供了一种方法,可以稳定地增强生物人工肝细胞中的功能基因表达,并具有大规模细胞扩增的潜力。











































 京公网安备 11010802027423号
京公网安备 11010802027423号