npj Parkinson's Disease ( IF 6.7 ) Pub Date : 2018-12-06 , DOI: 10.1038/s41531-018-0071-3 Per Svenningsson 1, 2 , Anders Johansson 1, 2 , Dag Nyholm 3 , Panagiota Tsitsi 1 , Fredrik Hansson 4 , Clas Sonesson 5 , Joakim Tedroff 1, 5
|
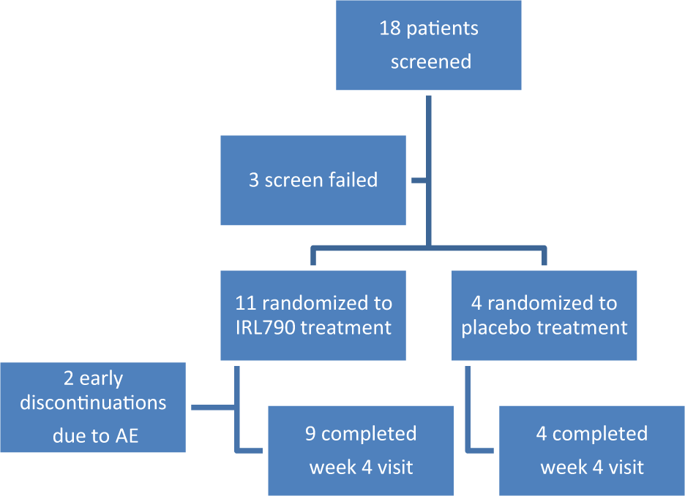
IRL790 is a novel compound with psychomotor stabilizing properties primarily targeting the dopamine D3 receptor. IRL790 is developed as an experimental treatment for levodopa-induced dyskinesia (LID), impulse control disorder, and psychosis in Parkinson’s disease (PD). The primary objective was to investigate the safety and tolerability of IRL790 in PD patients with LID in a randomized controlled trial. PD patients with peak-dose dyskinesia were randomized to placebo or IRL790 treatment (1:3 ratio) for 4 weeks. Study drug was given as an adjunct treatment to the patients’ regular stable antiparkinsonian medication. Dosing was individually titrated for 14 days, whereafter dosing was kept stable for an additional 14 days. Fifteen patients were randomized to treatment and 13 patients completed the 4-week treatment. Adverse events were mostly reported during the titration phase of the trial. They were mainly central nervous system related and could be mitigated by dose adjustments. There were no serious adverse events. There were no clinically significant changes in vital signs, electrocardiogram, and laboratory parameters due to the treatment. The average dose in the stable dose phase was 18 mg daily, yielding a 2-h post-dose plasma concentration of average 229 nM on day 28. Assessments for motor function showed a numeric reduction in dyskinesia. It is concluded that IRL790 can be safely administered to patients with advanced PD. The results will be of guidance for the design of phase 2 studies.
中文翻译:

IRL790 在帕金森病伴左旋多巴诱发的运动障碍中的安全性和耐受性——1b 期试验
IRL790 是一种新型化合物,具有精神运动稳定特性,主要针对多巴胺 D3 受体。 IRL790 被开发为一种实验性治疗药物,用于治疗左旋多巴引起的运动障碍 (LID)、冲动控制障碍和帕金森病 (PD) 中的精神病。主要目的是在一项随机对照试验中研究 IRL790 在患有 LID 的 PD 患者中的安全性和耐受性。患有峰值剂量运动障碍的 PD 患者被随机接受安慰剂或 IRL790 治疗(1:3 比例),为期 4 周。研究药物作为患者常规稳定抗帕金森病药物的辅助治疗。剂量在 14 天内单独滴定,此后剂量在另外 14 天内保持稳定。 15 名患者被随机接受治疗,13 名患者完成了 4 周的治疗。不良事件主要是在试验的滴定阶段报告的。它们主要与中枢神经系统有关,可以通过剂量调整来减轻。没有发生严重的不良事件。由于治疗,生命体征、心电图和实验室参数没有出现临床上显着的变化。稳定剂量阶段的平均剂量为每天 18 mg,第 28 天给药后 2 小时血浆浓度平均为 229 nM。运动功能评估显示运动障碍明显减少。结论是IRL790可以安全地用于晚期PD患者。结果将为第二阶段研究的设计提供指导。











































 京公网安备 11010802027423号
京公网安备 11010802027423号