当前位置:
X-MOL 学术
›
Acta Pharmacol. Sin.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Human pharmacology of positive GABA-A subtype-selective receptor modulators for the treatment of anxiety.
Acta Pharmacologica Sinica ( IF 6.9 ) Pub Date : 2018-12-05 , DOI: 10.1038/s41401-018-0185-5 Xia Chen 1, 2, 3, 4 , Joop van Gerven 4, 5 , Adam Cohen 4 , Gabriel Jacobs 4
Acta Pharmacologica Sinica ( IF 6.9 ) Pub Date : 2018-12-05 , DOI: 10.1038/s41401-018-0185-5 Xia Chen 1, 2, 3, 4 , Joop van Gerven 4, 5 , Adam Cohen 4 , Gabriel Jacobs 4
Affiliation
|
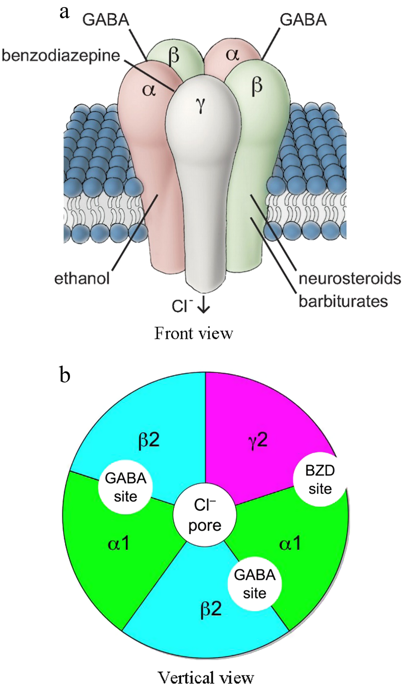
Anxiety disorders arise from disruptions among the highly interconnected circuits that normally serve to process the streams of potentially threatening stimuli. The resulting imbalance among these circuits can cause a fundamental misinterpretation of neural sensory information as threatening and can lead to the inappropriate emotional and behavioral responses observed in anxiety disorders. There is considerable preclinical evidence that the GABAergic system, in general, and its α2- and/or α5-subunit-containing GABA(A) receptor subtypes, in particular, are involved in the pathophysiology of anxiety disorders. However, the clinical efficacy of GABA-A α2-selective agonists for the treatment of anxiety disorders has not been unequivocally demonstrated. In this review, we present several human pharmacological studies that have been performed with the aim of identifying the pharmacologically active doses/exposure levels of several GABA-A subtype-selective novel compounds with potential anxiolytic effects. The pharmacological selectivity of novel α2-subtype-selective GABA(A) receptor partial agonists has been demonstrated by their distinct effect profiles on the neurophysiological and neuropsychological measurements that reflect the functions of multiple CNS domains compared with those of benzodiazepines, which are nonselective, full GABA(A) agonists. Normalizing the undesired pharmacodynamic side effects against the desired on-target effects on the saccadic peak velocity is a useful approach for presenting the pharmacological features of GABA(A)-ergic modulators. Moreover, combining the anxiogenic symptom provocation paradigm with validated neurophysiological and neuropsychological biomarkers may provide further construct validity for the clinical effects of novel anxiolytic agents. In addition, the observed drug effects on serum prolactin levels support the use of serum prolactin levels as a complementary neuroendocrine biomarker to further validate the pharmacodynamic measurements used during the clinical pharmacological study of novel anxiolytic agents.
中文翻译:

用于治疗焦虑症的阳性 GABA-A 亚型选择性受体调节剂的人体药理学。
焦虑障碍源于高度互连的回路之间的中断,这些回路通常用于处理潜在的威胁性刺激流。这些回路之间由此产生的不平衡会导致对神经感觉信息的根本误解为具有威胁性,并可能导致在焦虑症中观察到的不适当的情绪和行为反应。大量的临床前证据表明,一般而言,GABA 能系统及其含有 α2 和/或 α5 亚基的 GABA(A) 受体亚型与焦虑症的病理生理有关。然而,尚未明确证明 GABA-A α2 选择性激动剂治疗焦虑症的临床疗效。在这次审查中,我们介绍了几项人类药理学研究,这些研究旨在确定具有潜在抗焦虑作用的几种 GABA-A 亚型选择性新型化合物的药理活性剂量/暴露水平。新型 α2 亚型选择性 GABA(A) 受体部分激动剂的药理学选择性已通过它们对反映多个 CNS 域功能的神经生理学和神经心理学测量的独特影响特征得到证明,与苯二氮卓类相比,苯二氮卓类是非选择性的、完全的GABA(A) 激动剂。将不需要的药效学副作用与对扫视峰值速度的预期目标效应标准化是呈现 GABA(A) 能调节剂的药理学特征的有用方法。而且,将产生焦虑的症状激发范式与经过验证的神经生理学和神经心理学生物标志物相结合,可以为新型抗焦虑药的临床效果提供进一步的结构有效性。此外,观察到的药物对血清催乳素水平的影响支持使用血清催乳素水平作为补充的神经内分泌生物标志物,以进一步验证在新型抗焦虑药的临床药理学研究中使用的药效学测量。
更新日期:2019-05-16
中文翻译:

用于治疗焦虑症的阳性 GABA-A 亚型选择性受体调节剂的人体药理学。
焦虑障碍源于高度互连的回路之间的中断,这些回路通常用于处理潜在的威胁性刺激流。这些回路之间由此产生的不平衡会导致对神经感觉信息的根本误解为具有威胁性,并可能导致在焦虑症中观察到的不适当的情绪和行为反应。大量的临床前证据表明,一般而言,GABA 能系统及其含有 α2 和/或 α5 亚基的 GABA(A) 受体亚型与焦虑症的病理生理有关。然而,尚未明确证明 GABA-A α2 选择性激动剂治疗焦虑症的临床疗效。在这次审查中,我们介绍了几项人类药理学研究,这些研究旨在确定具有潜在抗焦虑作用的几种 GABA-A 亚型选择性新型化合物的药理活性剂量/暴露水平。新型 α2 亚型选择性 GABA(A) 受体部分激动剂的药理学选择性已通过它们对反映多个 CNS 域功能的神经生理学和神经心理学测量的独特影响特征得到证明,与苯二氮卓类相比,苯二氮卓类是非选择性的、完全的GABA(A) 激动剂。将不需要的药效学副作用与对扫视峰值速度的预期目标效应标准化是呈现 GABA(A) 能调节剂的药理学特征的有用方法。而且,将产生焦虑的症状激发范式与经过验证的神经生理学和神经心理学生物标志物相结合,可以为新型抗焦虑药的临床效果提供进一步的结构有效性。此外,观察到的药物对血清催乳素水平的影响支持使用血清催乳素水平作为补充的神经内分泌生物标志物,以进一步验证在新型抗焦虑药的临床药理学研究中使用的药效学测量。











































 京公网安备 11010802027423号
京公网安备 11010802027423号