当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
External Reversal of Chirality Transfer in Photoswitches
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2019-01-18 , DOI: 10.1002/anie.201812284 Christoph Jurissek 1 , Fabian Berger 1 , Fabian Eisenreich 1 , Michael Kathan 1 , Stefan Hecht 1
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2019-01-18 , DOI: 10.1002/anie.201812284 Christoph Jurissek 1 , Fabian Berger 1 , Fabian Eisenreich 1 , Michael Kathan 1 , Stefan Hecht 1
Affiliation
|
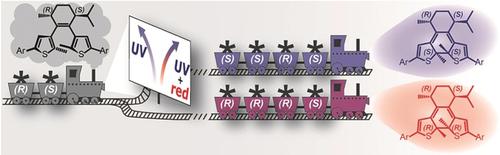
The transfer of stereoinformation is at the heart of asymmetric reactions. By incorporating the natural monoterpene l‐menthone into the backbone of a diarylethene, we achieved efficient chirality transfer upon photocyclization, resulting in the preferred formation of one major closed isomer in a diastereomeric ratio (d.r.) of 85:15. More significantly, we were able to completely reverse the diastereomeric outcome of the ring closure simply by altering the chemical environment or the irradiation conditions. As a result, we could selectively accumulate the less favored minor closed isomer, with remarkable d.r. values of >99:1 and 74:26, respectively. Computations revealed that a stability inversion after photocyclization is the basis for the observed unprecedented control over diastereoselectivity.
中文翻译:

光电开关中手性传递的外部逆转
立体信息的传递是不对称反应的核心。通过将天然单萜l-薄荷酮掺入二芳基乙烯的骨架中,我们在光环化后实现了有效的手性转移,从而导致以85:15的非对映异构体比率(dr)较好地形成了一种主要的封闭异构体。更重要的是,仅通过改变化学环境或辐照条件,我们就能够完全逆转闭环的非对映异构体结果。结果,我们可以有选择地累积不太受欢迎的次要封闭异构体,其dr值分别> 99:1和74:26。计算表明,光环化后的稳定性反转是观察到的对非对映选择性空前控制的基础。
更新日期:2019-01-18
中文翻译:

光电开关中手性传递的外部逆转
立体信息的传递是不对称反应的核心。通过将天然单萜l-薄荷酮掺入二芳基乙烯的骨架中,我们在光环化后实现了有效的手性转移,从而导致以85:15的非对映异构体比率(dr)较好地形成了一种主要的封闭异构体。更重要的是,仅通过改变化学环境或辐照条件,我们就能够完全逆转闭环的非对映异构体结果。结果,我们可以有选择地累积不太受欢迎的次要封闭异构体,其dr值分别> 99:1和74:26。计算表明,光环化后的稳定性反转是观察到的对非对映选择性空前控制的基础。











































 京公网安备 11010802027423号
京公网安备 11010802027423号