当前位置:
X-MOL 学术
›
Mol. Pharmaceutics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Impact of a Single Hydrogen Substitution by Fluorine on the Molecular Interaction and Miscibility between Sorafenib and Polymers
Molecular Pharmaceutics ( IF 4.5 ) Pub Date : 2018-12-04 00:00:00 , DOI: 10.1021/acs.molpharmaceut.8b00970 Chengyu Liu 1 , Cong-Qiao Xu 2 , Junguang Yu 1 , Yipshu Pui 1 , Huijun Chen 1 , Shan Wang 1 , Alan Donghua Zhu 3 , Jun Li 2 , Feng Qian 1
Molecular Pharmaceutics ( IF 4.5 ) Pub Date : 2018-12-04 00:00:00 , DOI: 10.1021/acs.molpharmaceut.8b00970 Chengyu Liu 1 , Cong-Qiao Xu 2 , Junguang Yu 1 , Yipshu Pui 1 , Huijun Chen 1 , Shan Wang 1 , Alan Donghua Zhu 3 , Jun Li 2 , Feng Qian 1
Affiliation
|
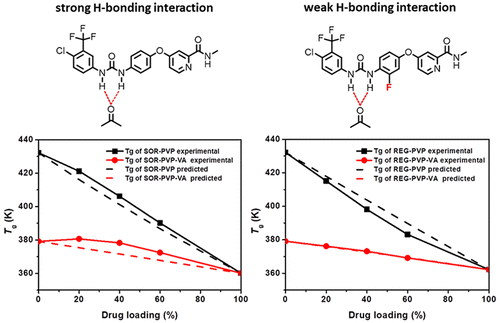
We aim to understand the potential impact of a modest chemical modification of a drug molecule on the downstream design of its amorphous solid dispersion (ASD) formulation. To this end, we used sorafenib (SOR) and its fluorinated form, regorafenib (REG), as model drugs, to assess the impact of a single hydrogen substitution by fluorine on the molecular interaction and miscibility between drug and PVP or PVP–VA, two commonly used polymers for ASDs. In this study, we observed that the Tg values of PVP or PVP–VA based ASDs of SOR deviated positively from the Gordon–Taylor prediction, which assumes ideal mixing, yet the Tg of REG ASDs deviated negatively from or matched well with the ideal mixing model, suggesting much stronger drug–polymer interactions in SOR ASDs compared with the REG ASDs. Using solution NMR and computational methods, we proved that a six-member-ring formed between the carbonyl groups on the polymers and the uramido hydrogen of SOR or REG, through intermolecular hydrogen bonding. However, steric hindrance resulting from fluorination in REG caused weaker interaction between REG–polymer than SOR–polymer. To further confirm this mechanism, we investigated the molecular interactions of other two uramido-containing model compounds, triclocarban (TCC) and gliclazide (GCZ), with PVP. We found that TCC but not GCZ formed a hexatomic ring with PVP. We concluded that PVP based polymers can easily interact with N,N′-disubstituted urea compounds with a trans–trans structure in the form of hexatomic rings, and the interaction strength of the hexatomic ring largely depended on the chemistry of drug molecules. This study illustrated that even a slight chemical modification on drug molecules could result in substantial difference in drug–polymer interactions, thus significantly impacting polymer selection and pharmaceutical performance of their ASD formulations.
中文翻译:

氟单氢取代对索拉非尼与聚合物之间分子相互作用和互溶性的影响
我们旨在了解药物分子适度化学修饰对其无定形固体分散体(ASD)制剂下游设计的潜在影响。为此,我们使用索拉非尼(SOR)及其氟化形式雷戈非尼(REG)作为模型药物,来评估氟单氢取代对药物与PVP或PVP-VA之间的分子相互作用和互溶性的影响,两种用于ASD的常用聚合物。在这项研究中,我们观察到Ť克PVP或PVP-VA基于SOR的自闭症的值从戈登-泰勒预测,其假设理想的混合,但正偏移Ť克REG ASD的偏离理想混合模型或与理想混合模型良好匹配,表明与REG ASD相比,SOR ASD中的药物-聚合物相互作用更强。使用溶液核磁共振和计算方法,我们证明了通过分子间氢键在聚合物上的羰基与SOR或REG的尿嘧啶氢之间形成了六元环。但是,REG中氟化引起的空间位阻导致REG聚合物之间的相互作用比SOR聚合物弱。为了进一步证实该机制,我们研究了其他两种含铀酰氨基的模型化合物三氯卡班(TCC)和格列齐特(GCZ)与PVP的分子相互作用。我们发现,TCC而非GCZ与PVP形成了一个六环。我们得出的结论是,基于PVP的聚合物可以轻松地与N相互作用,N'-二取代脲化合物具有六环形式的反式-反式结构,六环的相互作用强度在很大程度上取决于药物分子的化学性质。这项研究表明,即使对药物分子进行微小的化学修饰,也可能导致药物与聚合物之间的相互作用产生实质性差异,从而显着影响其ASD制剂的聚合物选择和药物性能。
更新日期:2018-12-04
中文翻译:

氟单氢取代对索拉非尼与聚合物之间分子相互作用和互溶性的影响
我们旨在了解药物分子适度化学修饰对其无定形固体分散体(ASD)制剂下游设计的潜在影响。为此,我们使用索拉非尼(SOR)及其氟化形式雷戈非尼(REG)作为模型药物,来评估氟单氢取代对药物与PVP或PVP-VA之间的分子相互作用和互溶性的影响,两种用于ASD的常用聚合物。在这项研究中,我们观察到Ť克PVP或PVP-VA基于SOR的自闭症的值从戈登-泰勒预测,其假设理想的混合,但正偏移Ť克REG ASD的偏离理想混合模型或与理想混合模型良好匹配,表明与REG ASD相比,SOR ASD中的药物-聚合物相互作用更强。使用溶液核磁共振和计算方法,我们证明了通过分子间氢键在聚合物上的羰基与SOR或REG的尿嘧啶氢之间形成了六元环。但是,REG中氟化引起的空间位阻导致REG聚合物之间的相互作用比SOR聚合物弱。为了进一步证实该机制,我们研究了其他两种含铀酰氨基的模型化合物三氯卡班(TCC)和格列齐特(GCZ)与PVP的分子相互作用。我们发现,TCC而非GCZ与PVP形成了一个六环。我们得出的结论是,基于PVP的聚合物可以轻松地与N相互作用,N'-二取代脲化合物具有六环形式的反式-反式结构,六环的相互作用强度在很大程度上取决于药物分子的化学性质。这项研究表明,即使对药物分子进行微小的化学修饰,也可能导致药物与聚合物之间的相互作用产生实质性差异,从而显着影响其ASD制剂的聚合物选择和药物性能。











































 京公网安备 11010802027423号
京公网安备 11010802027423号