当前位置:
X-MOL 学术
›
ACS Cent. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Acyl Glycosides through Stereospecific Glycosyl Cross-Coupling: Rapid Access to C(sp3)-Linked Glycomimetics
ACS Central Science ( IF 12.7 ) Pub Date : 2018-12-04 00:00:00 , DOI: 10.1021/acscentsci.8b00628 Feng Zhu 1 , Jacob Rodriguez 1 , Sloane O'Neill 1 , Maciej A Walczak 1
ACS Central Science ( IF 12.7 ) Pub Date : 2018-12-04 00:00:00 , DOI: 10.1021/acscentsci.8b00628 Feng Zhu 1 , Jacob Rodriguez 1 , Sloane O'Neill 1 , Maciej A Walczak 1
Affiliation
|
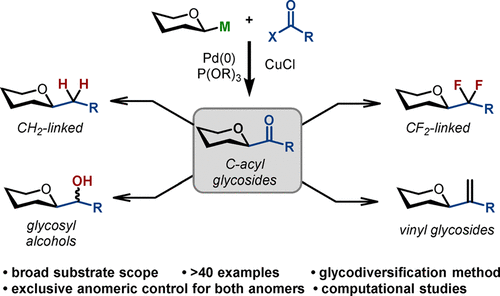
Replacement of a glycosidic bond with hydrolytically stable C–C surrogates is an efficient strategy to access glycomimetics with improved physicochemical and pharmacological properties. We describe here a stereoretentive cross-coupling reaction of glycosyl stannanes with C(sp2)- and C(sp3)-thio(seleno)esters suitable for the preparation C-acyl glycosides as synthetic building blocks to obtain C(sp3)-linked and fluorinated glycomimetics. First, we identified a set of standardized conditions employing a Pd(0) precatalyst, CuCl additive, and phosphite ligand that provided access to C-acyl glycosides without deterioration of anomeric integrity and decarbonylation of the acyl donors (>40 examples). Second, we demonstrated that C(sp3)-glycomimetics could be introduced into the anomeric position via a direct conversion of C1 ketones. Specifically, the conversion of the carbonyl group into a CF2 mimetic is an appealing method to access valuable fluorinated analogues. We also illustrate that the introduction of other carbonyl-based groups into the C1 position of mono- and oligosaccharides can be accomplished using the corresponding acyl donors. This protocol is amenable to late-stage glycodiversification and programmed mutation of the C–O bond into hydrolytically stable C–C bonds. Taken together, stereoretentive anomeric acylation represents a convenient method to prepare a diverse set of glycan mimetics with minimal synthetic manipulations and with absolute control of anomeric configuration.
中文翻译:

通过立体特异性糖基交叉偶联的酰基糖苷:快速获得 C(sp3)-连接的糖模拟物
用水解稳定的 C-C 替代物取代糖苷键是获得具有改善的物理化学和药理学特性的糖模拟物的有效策略。我们在此描述了糖基锡烷与 C(sp 2 )-和 C(sp 3 )-硫代(硒)酯的立体保留交叉偶联反应,该反应适合制备C-酰基糖苷作为合成结构单元以获得 C(sp 3 ) -连接的和氟化的糖模拟物。首先,我们确定了一组使用 Pd(0) 预催化剂、CuCl 添加剂和亚磷酸酯配体的标准化条件,这些条件提供了获得C-酰基糖苷的途径,而不会破坏异头完整性和酰基供体的脱羰作用(> 40 个示例)。其次,我们证明了 C(sp 3 )-糖模拟物可以通过 C1 酮的直接转化引入异头位置。具体而言,将羰基转化为CF 2模拟物是获得有价值的氟化类似物的一种有吸引力的方法。我们还说明,可以使用相应的酰基供体将其他羰基基团引入单糖和寡糖的 C1 位。该方案适合后期糖多样化和 C-O 键编程突变为水解稳定的 C-C 键。总而言之,立体保留异头酰化代表了一种用最少的合成操作和对异头构型的绝对控制来制备多种聚糖模拟物的便捷方法。
更新日期:2018-12-04
中文翻译:

通过立体特异性糖基交叉偶联的酰基糖苷:快速获得 C(sp3)-连接的糖模拟物
用水解稳定的 C-C 替代物取代糖苷键是获得具有改善的物理化学和药理学特性的糖模拟物的有效策略。我们在此描述了糖基锡烷与 C(sp 2 )-和 C(sp 3 )-硫代(硒)酯的立体保留交叉偶联反应,该反应适合制备C-酰基糖苷作为合成结构单元以获得 C(sp 3 ) -连接的和氟化的糖模拟物。首先,我们确定了一组使用 Pd(0) 预催化剂、CuCl 添加剂和亚磷酸酯配体的标准化条件,这些条件提供了获得C-酰基糖苷的途径,而不会破坏异头完整性和酰基供体的脱羰作用(> 40 个示例)。其次,我们证明了 C(sp 3 )-糖模拟物可以通过 C1 酮的直接转化引入异头位置。具体而言,将羰基转化为CF 2模拟物是获得有价值的氟化类似物的一种有吸引力的方法。我们还说明,可以使用相应的酰基供体将其他羰基基团引入单糖和寡糖的 C1 位。该方案适合后期糖多样化和 C-O 键编程突变为水解稳定的 C-C 键。总而言之,立体保留异头酰化代表了一种用最少的合成操作和对异头构型的绝对控制来制备多种聚糖模拟物的便捷方法。











































 京公网安备 11010802027423号
京公网安备 11010802027423号