当前位置:
X-MOL 学术
›
ACS Chem. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Extender Unit Promiscuity and Orthogonal Protein Interactions of an Aminomalonyl-ACP Utilizing Trans-Acyltransferase from Zwittermicin Biosynthesis.
ACS Chemical Biology ( IF 3.5 ) Pub Date : 2018-11-28 , DOI: 10.1021/acschembio.8b00867 Samantha M Carpenter 1 , Gavin J Williams 1, 2
ACS Chemical Biology ( IF 3.5 ) Pub Date : 2018-11-28 , DOI: 10.1021/acschembio.8b00867 Samantha M Carpenter 1 , Gavin J Williams 1, 2
Affiliation
|
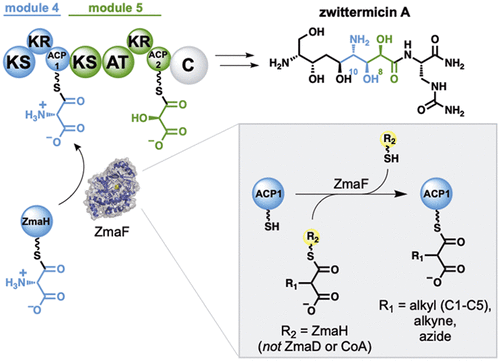
Trans-acting acyltransferases (trans-ATs) are standalone enzymes that select and deliver extender units to polyketide synthase assembly lines. Accordingly, there is interest in leveraging trans-ATs as tools to regioselectively diversify polyketide structures. Yet, little is known regarding the extender unit and acyl carrier protein (ACP) specificity of trans-ATs, particularly those that utilize unusual ACP-linked extender units. For example, the biosynthesis of the antibiotic zwittermicin involves the trans-AT ZmaF, which is responsible for installing a rare ACP-linked aminomalonyl extender unit. Here, we developed a method to access a panel of non-natural and non-native ACP-linked extender units and used it to probe the promiscuity of ZmaF, revealing one of the most promiscuous ATs characterized to date. Furthermore, we demonstrated that ZmaF is highly orthogonal with respect to its ACP specificity, and the ability of ZmaF to trans-complement noncognate PKS modules was also explored. Together, these results set the stage for further engineering ZmaF as a tool for polyketide diversification.
中文翻译:

利用Zwittermicin生物合成中反式酰基转移酶的氨纶-ACP的延伸单位混杂和正交蛋白质相互作用。
反式酰基转移酶(trans-ATs)是独立的酶,可以选择并向聚酮化合物合酶装配线提供扩展单元。因此,有兴趣利用反式-AT作为工具来使聚酮化合物结构区域选择性地多样化。然而,关于反式AT的延伸单元和酰基载体蛋白(ACP)的特异性知之甚少,特别是那些利用不寻常的ACP连接的延伸单元的反式AT。例如,抗生素茨维特霉素的生物合成涉及反式-AT ZmaF,其负责安装稀有的ACP连接的氨基丙二酸增量剂单元。在这里,我们开发了一种方法来访问一组非天然和非天然ACP链接的扩展单元,并用它来探测ZmaF的混杂,揭示了迄今为止特征最复杂的AT之一。此外,我们证明ZmaF就其ACP特异性而言是高度正交的,并且还探索了ZmaF反互补非同源PKS模块的能力。总之,这些结果为进一步设计ZmaF作为聚酮化合物多样化的工具奠定了基础。
更新日期:2018-11-28
中文翻译:

利用Zwittermicin生物合成中反式酰基转移酶的氨纶-ACP的延伸单位混杂和正交蛋白质相互作用。
反式酰基转移酶(trans-ATs)是独立的酶,可以选择并向聚酮化合物合酶装配线提供扩展单元。因此,有兴趣利用反式-AT作为工具来使聚酮化合物结构区域选择性地多样化。然而,关于反式AT的延伸单元和酰基载体蛋白(ACP)的特异性知之甚少,特别是那些利用不寻常的ACP连接的延伸单元的反式AT。例如,抗生素茨维特霉素的生物合成涉及反式-AT ZmaF,其负责安装稀有的ACP连接的氨基丙二酸增量剂单元。在这里,我们开发了一种方法来访问一组非天然和非天然ACP链接的扩展单元,并用它来探测ZmaF的混杂,揭示了迄今为止特征最复杂的AT之一。此外,我们证明ZmaF就其ACP特异性而言是高度正交的,并且还探索了ZmaF反互补非同源PKS模块的能力。总之,这些结果为进一步设计ZmaF作为聚酮化合物多样化的工具奠定了基础。











































 京公网安备 11010802027423号
京公网安备 11010802027423号