当前位置:
X-MOL 学术
›
Mol. Pharmaceutics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
In Situ Characterization of the Microstructural Evolution of Biopharmaceutical Solid-State Formulations with Implications for Protein Stability
Molecular Pharmaceutics ( IF 4.5 ) Pub Date : 2018-11-28 00:00:00 , DOI: 10.1021/acs.molpharmaceut.8b00935 Stijn H. S. Koshari 1 , Purnendu K. Nayak 2 , Shalini Burra 2 , Isidro E. Zarraga , Karthikan Rajagopal , Yun Liu 1, 3 , Norman J. Wagner 1 , Abraham M. Lenhoff 1
Molecular Pharmaceutics ( IF 4.5 ) Pub Date : 2018-11-28 00:00:00 , DOI: 10.1021/acs.molpharmaceut.8b00935 Stijn H. S. Koshari 1 , Purnendu K. Nayak 2 , Shalini Burra 2 , Isidro E. Zarraga , Karthikan Rajagopal , Yun Liu 1, 3 , Norman J. Wagner 1 , Abraham M. Lenhoff 1
Affiliation
|
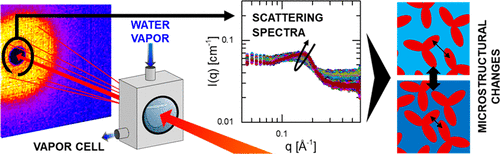
Lyophilized and spray-dried biopharmaceutical formulations are used to provide long-term stability for storage and transport, but questions remain about the molecular structure in these solid formulations and how this structure may be responsible for protein stability. Small-angle neutron scattering with a humidity control environment is used to characterize protein-scale microstructural changes in such solid-state formulations as they are humidified and dried in situ. The findings indicate that irreversible protein aggregates of stressed formulations do not form within the solid-state but do emerge upon reconstitution of the formulation. After plasticization of the solid-state matrix by exposure to humidity, the formation of reversibly self-associating aggregates can be detected in situ. The characterization of the protein-scale microstructure in these solid-state formulations facilitates further efforts to understand the underlying mechanisms that promote long-term protein stability.
中文翻译:

原位表征的生物制药固态制剂的微观结构演变对蛋白质稳定性的影响
冻干和喷雾干燥的生物药物制剂用于提供储存和运输的长期稳定性,但是有关这些固体制剂中的分子结构以及这种结构如何影响蛋白质稳定性的问题仍然存在。使用具有湿度控制环境的小角度中子散射来表征固态配方中蛋白质的微观结构变化,因为这些固态配方被原位加湿和干燥。研究结果表明,应激制剂的不可逆蛋白聚集体不会在固态内形成,但会在重构后出现。通过暴露在湿气中使固态基质增塑后,可就地检测到可逆自缔合聚集体的形成。
更新日期:2018-11-28
中文翻译:

原位表征的生物制药固态制剂的微观结构演变对蛋白质稳定性的影响
冻干和喷雾干燥的生物药物制剂用于提供储存和运输的长期稳定性,但是有关这些固体制剂中的分子结构以及这种结构如何影响蛋白质稳定性的问题仍然存在。使用具有湿度控制环境的小角度中子散射来表征固态配方中蛋白质的微观结构变化,因为这些固态配方被原位加湿和干燥。研究结果表明,应激制剂的不可逆蛋白聚集体不会在固态内形成,但会在重构后出现。通过暴露在湿气中使固态基质增塑后,可就地检测到可逆自缔合聚集体的形成。











































 京公网安备 11010802027423号
京公网安备 11010802027423号