当前位置:
X-MOL 学术
›
Mol. Pharmaceutics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Influence of Drug–Polymer Interactions on Dissolution of Thermodynamically Highly Unstable Cocrystal
Molecular Pharmaceutics ( IF 4.5 ) Pub Date : 2018-11-27 00:00:00 , DOI: 10.1021/acs.molpharmaceut.8b00923 Milankumar S. Jasani 1 , Dnyaneshwar P. Kale 1 , Inder Pal Singh , Arvind K. Bansal 1
Molecular Pharmaceutics ( IF 4.5 ) Pub Date : 2018-11-27 00:00:00 , DOI: 10.1021/acs.molpharmaceut.8b00923 Milankumar S. Jasani 1 , Dnyaneshwar P. Kale 1 , Inder Pal Singh , Arvind K. Bansal 1
Affiliation
|
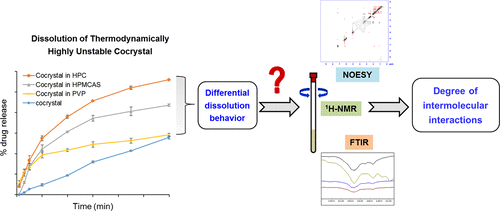
Solubility advantage of thermodynamically highly unstable cocrystals, which undergo solution-mediated phase transformation (SMPT) in less than 1 min, does not translate to enhanced dissolution. The present study was aimed to understand the impact of polymeric additives on dissolution of thermodynamically highly unstable cocrystal with specific emphasis on influence of drug–polymer interactions. Exemestane-maleic acid was selected as a model cocrystal with SMPT time of <30 s and eutectic constant (Keu) of 75475. Hydroxypropylcellulose (HPC), hydroxypropyl methylcellulose acetate succinate (HPMCAS), and polyvinylpyrrolidone (PVP) were selected as polymers for a dissolution study based on measurement of induction time using precipitation study. In the presence of 0.2% w/v of HPC, the cocrystal showed significantly higher drug release (∼3-fold) as compared with the cocrystal in the absence of predissolved polymers. Differential dissolution profiles of the cocrystal were observed with each polymer and the order of increasing dissolution rate was found to be HPC ≈ HPMCAS > PVP. The molecular basis of the differential dissolution performance was investigated using infrared spectroscopy, solution-state nuclear magnetic resonance spectroscopy, and nuclear Overhauser effect spectroscopy (NOESY). The polymers with stronger interactions with drug in the cocrystal (HPMCAS and HPC) displayed higher dissolution rate as compared with that of no intermolecular interaction (PVP). The study also highlighted that, despite no influence of the polymers on the cocrystal SMPT, dissolution enhancement was achieved. This was attributed to small-sized drug crystals (1–3 μm) generated from the supersaturation-mediated crystallization and improved solvation due to drug–polymer interactions. These findings have implications on development of drug products using thermodynamically unstable cocrystals.
中文翻译:

药物-聚合物相互作用对热力学上高度不稳定的共晶溶解的影响
热力学上高度不稳定的共晶在不到1分钟的时间内经历溶液介导的相变(SMPT)的溶解度优势并未转化为增强的溶解度。本研究的目的是了解聚合物添加剂对热力学高度不稳定的共晶溶解的影响,并特别强调药物-聚合物相互作用的影响。选择依西美坦-马来酸为模型共晶,SMPT时间<30 s,共晶常数(K eu根据沉淀法测量诱导时间,选择羟丙基纤维素(HPC),羟丙基甲基纤维素乙酸琥珀酸酯(HPMCAS)和聚乙烯吡咯烷酮(PVP)作为溶出研究的聚合物。在存在0.2%w / v的HPC的情况下,与不存在预溶解的聚合物的情况下相比,共晶体显示出明显更高的药物释放(约3倍)。观察到每种聚合物共晶的不同溶出曲线,发现溶出速率增加的顺序为HPC≈HPMCAS> PVP。使用红外光谱,溶液态核磁共振光谱和核Overhauser效应光谱(NOESY)研究了差异溶解性能的分子基础。与没有分子间相互作用(PVP)的聚合物相比,在共晶体中与药物相互作用更强的聚合物(HPMCAS和HPC)显示出更高的溶出速率。该研究还强调,尽管聚合物对共晶SMPT没有影响,但溶解度却得以提高。这归因于过饱和介导的结晶产生的小尺寸药物晶体(1-3μm),以及由于药物-聚合物相互作用而改善了溶剂化作用。这些发现对使用热力学不稳定的共晶体开发药物产品具有重要意义。这归因于过饱和介导的结晶产生的小尺寸药物晶体(1-3μm),以及由于药物-聚合物相互作用而改善了溶剂化作用。这些发现对使用热力学不稳定的共晶体开发药物产品具有重要意义。这归因于过饱和介导的结晶产生的小尺寸药物晶体(1-3μm),以及由于药物-聚合物相互作用而改善了溶剂化作用。这些发现对使用热力学不稳定的共晶体开发药物产品具有重要意义。
更新日期:2018-11-27
中文翻译:

药物-聚合物相互作用对热力学上高度不稳定的共晶溶解的影响
热力学上高度不稳定的共晶在不到1分钟的时间内经历溶液介导的相变(SMPT)的溶解度优势并未转化为增强的溶解度。本研究的目的是了解聚合物添加剂对热力学高度不稳定的共晶溶解的影响,并特别强调药物-聚合物相互作用的影响。选择依西美坦-马来酸为模型共晶,SMPT时间<30 s,共晶常数(K eu根据沉淀法测量诱导时间,选择羟丙基纤维素(HPC),羟丙基甲基纤维素乙酸琥珀酸酯(HPMCAS)和聚乙烯吡咯烷酮(PVP)作为溶出研究的聚合物。在存在0.2%w / v的HPC的情况下,与不存在预溶解的聚合物的情况下相比,共晶体显示出明显更高的药物释放(约3倍)。观察到每种聚合物共晶的不同溶出曲线,发现溶出速率增加的顺序为HPC≈HPMCAS> PVP。使用红外光谱,溶液态核磁共振光谱和核Overhauser效应光谱(NOESY)研究了差异溶解性能的分子基础。与没有分子间相互作用(PVP)的聚合物相比,在共晶体中与药物相互作用更强的聚合物(HPMCAS和HPC)显示出更高的溶出速率。该研究还强调,尽管聚合物对共晶SMPT没有影响,但溶解度却得以提高。这归因于过饱和介导的结晶产生的小尺寸药物晶体(1-3μm),以及由于药物-聚合物相互作用而改善了溶剂化作用。这些发现对使用热力学不稳定的共晶体开发药物产品具有重要意义。这归因于过饱和介导的结晶产生的小尺寸药物晶体(1-3μm),以及由于药物-聚合物相互作用而改善了溶剂化作用。这些发现对使用热力学不稳定的共晶体开发药物产品具有重要意义。这归因于过饱和介导的结晶产生的小尺寸药物晶体(1-3μm),以及由于药物-聚合物相互作用而改善了溶剂化作用。这些发现对使用热力学不稳定的共晶体开发药物产品具有重要意义。











































 京公网安备 11010802027423号
京公网安备 11010802027423号