当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Solid–Liquid Equilibrium of the Quaternary System Ca2+, Mg2+//SO32–, SO42–-H2O at 298.15 K
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2018-11-27 , DOI: 10.1021/acs.jced.8b00385 Xianze Meng 1 , Xiaozhen Wu 1 , Junsheng Yuan 1, 2
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2018-11-27 , DOI: 10.1021/acs.jced.8b00385 Xianze Meng 1 , Xiaozhen Wu 1 , Junsheng Yuan 1, 2
Affiliation
|
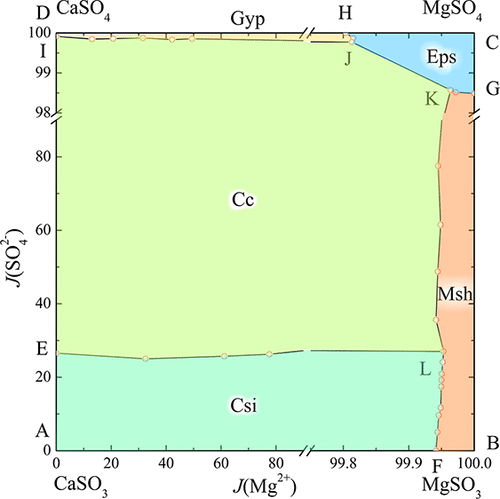
Solid–liquid equilibria of the quaternary system (Ca2+, Mg2+//SO32–, SO42–-H2O) and its ternary systems (Ca2+//SO32–, SO42–-H2O; Mg2+//SO32–, SO42–-H2O and Ca2+, Mg2+//SO32–-H2O) at 298.15 K were investigated via isothermal solution saturation method. Furthermore, the phase diagrams of these systems were constructed. The results show that the quaternary diagram contains three invariant points, seven univariant curves, and five crystallization fields corresponding to MgSO4·7H2O, MgSO3·6H2O, CaSO3·0.5H2O, Ca(SO3)0.89(SO4)0.11·0.5H2O, and CaSO4·2H2O. The crystallization field of Ca(SO3)0.89(SO4)0.11·0.5H2O is the largest compared with the others. In addition, crystal complex Ca(SO3)0.89(SO4)0.11·0.5H2O also appears in the ternary system (Ca2+//SO32–, SO42–-H2O), which contains two invariant points, three invariant curves, and three crystallization fields. Both of the other two ternary diagrams contain one invariant point, two invariant curves, and two crystallization fields. The Pitzer model was applied to calculate salt solubilities, and the results agree well with the experimental data. Binary and ternary ionic interaction parameters (θSO4,SO3, ψMg,SO4,SO3, ψCa,SO4,SO3, and ψCa,Mg,SO3) and ion pair parameters (CaSO3 and MgSO3) are presented in this work based on the solubilities of these ternary systems.
中文翻译:

298.15 K时四元系Ca 2 +,Mg 2+ // SO 3 2–,SO 4 2– -H 2 O的固液平衡
四元体系(Ca 2 +,Mg 2+ // SO 3 2–,SO 4 2– H 2 O)及其三元体系的固液平衡(Ca 2+ // SO 3 2–,SO 4 2 – -H 2 O; Mg 2+ // SO 3 2–,SO 4 2– -H 2 O和Ca 2 +,Mg 2+ // SO 3 2– -H 2通过等温溶液饱和法研究了298.15 K下的O)。此外,构建了这些系统的相图。结果表明,四元图包含三个不变点,七个单变曲线和五个结晶场,分别对应于MgSO 4 ·7H 2 O,MgSO 3 ·6H 2 O,CaSO 3 ·0.5H 2 O,Ca(SO 3)0.89(SO 4)0.11 ·0.5H 2 O,和的CaSO 4 ·2H 2 O.钙的结晶场(SO 3)0.89(SO 4)0.11 ·0.5H 2 O最大。此外,三元体系中还出现了晶体复合物Ca(SO 3)0.89(SO 4)0.11 ·0.5H 2 O(Ca 2+ // SO 3 2–,SO 4 2– -H 2O),其中包含两个不变点,三个不变曲线和三个结晶场。其他两个三元图都包含一个不变点,两个不变曲线和两个结晶场。用Pitzer模型计算盐的溶解度,其结果与实验数据吻合良好。本文介绍了二元和三元离子相互作用参数(θSO4,SO3,ψMg ,SO4,SO3,ψCa ,SO4,SO3和ψCa ,Mg,SO3)和离子对参数(CaSO 3和MgSO 3)基于这些三元系统的溶解度。
更新日期:2018-11-28
中文翻译:

298.15 K时四元系Ca 2 +,Mg 2+ // SO 3 2–,SO 4 2– -H 2 O的固液平衡
四元体系(Ca 2 +,Mg 2+ // SO 3 2–,SO 4 2– H 2 O)及其三元体系的固液平衡(Ca 2+ // SO 3 2–,SO 4 2 – -H 2 O; Mg 2+ // SO 3 2–,SO 4 2– -H 2 O和Ca 2 +,Mg 2+ // SO 3 2– -H 2通过等温溶液饱和法研究了298.15 K下的O)。此外,构建了这些系统的相图。结果表明,四元图包含三个不变点,七个单变曲线和五个结晶场,分别对应于MgSO 4 ·7H 2 O,MgSO 3 ·6H 2 O,CaSO 3 ·0.5H 2 O,Ca(SO 3)0.89(SO 4)0.11 ·0.5H 2 O,和的CaSO 4 ·2H 2 O.钙的结晶场(SO 3)0.89(SO 4)0.11 ·0.5H 2 O最大。此外,三元体系中还出现了晶体复合物Ca(SO 3)0.89(SO 4)0.11 ·0.5H 2 O(Ca 2+ // SO 3 2–,SO 4 2– -H 2O),其中包含两个不变点,三个不变曲线和三个结晶场。其他两个三元图都包含一个不变点,两个不变曲线和两个结晶场。用Pitzer模型计算盐的溶解度,其结果与实验数据吻合良好。本文介绍了二元和三元离子相互作用参数(θSO4,SO3,ψMg ,SO4,SO3,ψCa ,SO4,SO3和ψCa ,Mg,SO3)和离子对参数(CaSO 3和MgSO 3)基于这些三元系统的溶解度。











































 京公网安备 11010802027423号
京公网安备 11010802027423号