当前位置:
X-MOL 学术
›
Chem. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Cyclopentannulation and cyclodehydrogenation of isomerically pure 5,11-dibromo-anthradithiophenes leading to contorted aromatics†
Chemical Communications ( IF 4.3 ) Pub Date : 2018-11-23 00:00:00 , DOI: 10.1039/c8cc07327a Sambasiva R. Bheemireddy 1, 2, 3 , Waseem A. Hussain 1, 2, 3 , Ain Uddin 1, 2, 3 , Yachu Du 1, 2, 3 , Matthew P. Hautzinger 1, 2, 3 , Paul V. Kevorkian 1, 2, 3 , Frankie A. Petrie 1, 2, 3 , Kyle N. Plunkett 1, 2, 3
Chemical Communications ( IF 4.3 ) Pub Date : 2018-11-23 00:00:00 , DOI: 10.1039/c8cc07327a Sambasiva R. Bheemireddy 1, 2, 3 , Waseem A. Hussain 1, 2, 3 , Ain Uddin 1, 2, 3 , Yachu Du 1, 2, 3 , Matthew P. Hautzinger 1, 2, 3 , Paul V. Kevorkian 1, 2, 3 , Frankie A. Petrie 1, 2, 3 , Kyle N. Plunkett 1, 2, 3
Affiliation
|
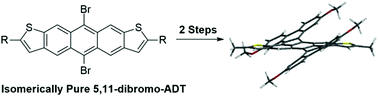
Isomerically pure 5,11-dibromo-2,8-dihexylanthra[2,3-b:76-b′]dithiophene, a brominated analog of anthracenedithiophene (ADT), was prepared and utilized for a palladium catalyzed cyclopentannulation reaction with 3,3′-dimethoxy-phenylacetylene to give cyclopentannulated ADT (CP-ADTs). A further Scholl cyclodehydrogenation reaction gave contorted aromatics with large splay angles, low optical gaps, and low LUMOs.
中文翻译:

异构体纯的5,11-二溴-蒽噻吩的环戊烷化和环脱氢反应导致芳族化合物的变形†
制备了异构的纯5,11-二溴-2,8-二己基噻吩[2,3- b:76- b ']二噻吩,一种溴化的蒽二噻吩(ADT)类似物,并用于钯催化的3,3环戊环化反应′-二甲氧基-苯基乙炔,得到环戊环化的ADT(CP-ADTs)。进一步的Scholl环脱氢反应得到扭曲的芳烃,其具有大张角,低光学间隙和低LUMO。
更新日期:2018-11-23
中文翻译:

异构体纯的5,11-二溴-蒽噻吩的环戊烷化和环脱氢反应导致芳族化合物的变形†
制备了异构的纯5,11-二溴-2,8-二己基噻吩[2,3- b:76- b ']二噻吩,一种溴化的蒽二噻吩(ADT)类似物,并用于钯催化的3,3环戊环化反应′-二甲氧基-苯基乙炔,得到环戊环化的ADT(CP-ADTs)。进一步的Scholl环脱氢反应得到扭曲的芳烃,其具有大张角,低光学间隙和低LUMO。











































 京公网安备 11010802027423号
京公网安备 11010802027423号