Catalysis Today ( IF 5.2 ) Pub Date : 2018-11-22 , DOI: 10.1016/j.cattod.2018.11.036 C. Hernández Mejía , C. Vogt , B.M. Weckhuysen , K.P. de Jong
|
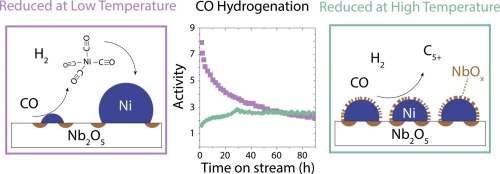
Stability of metal nanoparticles under reaction conditions is crucial in many catalytic processes. Nickel-based catalysts often encounter severe particle growth in the presence of carbon monoxide due to the formation and migration of nickel carbonyl. In this research, we showed that the reduction temperature of nickel oxide supported on niobia (Nb2O5) influenced the stability of the resulting nickel catalyst during subsequent carbon monoxide hydrogenation. Low reduction temperatures resulted in high initial nickel-normalized activity towards long-chain hydrocarbons (C5+), but fast deactivation throughout the experiment. High reduction temperatures led to a shift in product distribution towards shorter hydrocarbons and a decreased initial nickel-normalized activity, while during the first hours of the experiment an increase in turnover frequency and nickel-normalized activity was observed, resulting eventually in a stable catalytic performance. Electron microscopy analysis revealed extensive particle growth after catalysis when the catalyst had been reduced at low temperatures and no significant changes in particle size when reduced at high temperatures. By use of in-situ FT-IR spectroscopy, nickel subcarbonyl species which are precursors of volatile nickel tetracarbonyl were detected on Ni/Nb2O5 after low temperature reduction and exposure to CO, but not after high temperature reduction. Hence, particle growth is explained by the formation and diffusion of nickel carbonyl and subsequent Ostwald ripening, that leads to larger nickel particles with concomitant decrease in nickel-normalized activity. The stability of the catalyst reduced at high temperature was linked to the formation of niobium suboxides and their partial coverage of the nickel particles limiting the formation of nickel carbonyl and slowing down particle growth.
中文翻译:

稳定的由氧化铌负载的镍催化剂,用于一氧化碳加氢成烃
在许多催化过程中,金属纳米颗粒在反应条件下的稳定性至关重要。由于羰基镍的形成和迁移,镍基催化剂在一氧化碳的存在下经常会遇到严重的颗粒生长。在这项研究中,我们表明,负载在氧化铌上的氧化镍(Nb 2 O 5)的还原温度在随后的一氧化碳加氢过程中会影响所得镍催化剂的稳定性。较低的还原温度导致对长链碳氢化合物(C 5+),但在整个实验过程中会快速停用。较高的还原温度导致产物分布向较短的碳氢化合物转移,并且初始镍标准化活性降低,而在实验的最初几个小时内,观察到周转频率和镍标准化活性的增加,最终导致了稳定的催化性能。 。电子显微镜分析显示,当催化剂在低温下还原时,催化后颗粒会大量生长,而在高温下还原时,粒径没有明显变化。通过原位FT-IR光谱法,在Ni / Nb 2 O 5上检测到亚羰基镍是挥发性四羰基镍的前体。低温还原并暴露于CO后,但高温还原后则不然。因此,通过羰基镍的形成和扩散以及随后的奥斯特瓦尔德熟化来解释颗粒的生长,这导致更大的镍颗粒,同时镍标准化活性降低。在高温下还原的催化剂的稳定性与低价铌的形成有关,并且它们部分覆盖镍颗粒限制了羰基镍的形成并减慢了颗粒的生长。











































 京公网安备 11010802027423号
京公网安备 11010802027423号