当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Total Synthesis of Proanthocyanidin A1, A2, and Their Stereoisomers
Organic Letters ( IF 5.2 ) Pub Date : 2015-04-30 00:00:00 , DOI: 10.1021/acs.orglett.5b00646 Pradeep K. Sharma 1 , Leo J. Romanczyk 2 , Leelakrishna Kondaveti 1 , Bollu Reddy 1 , Jeeva Arumugasamy 1 , Richard Lombardy 1 , Yanni Gou 1 , Hagen Schroeter 2
Organic Letters ( IF 5.2 ) Pub Date : 2015-04-30 00:00:00 , DOI: 10.1021/acs.orglett.5b00646 Pradeep K. Sharma 1 , Leo J. Romanczyk 2 , Leelakrishna Kondaveti 1 , Bollu Reddy 1 , Jeeva Arumugasamy 1 , Richard Lombardy 1 , Yanni Gou 1 , Hagen Schroeter 2
Affiliation
|
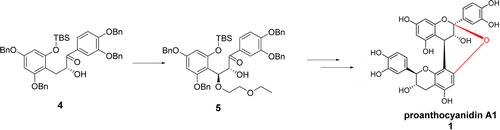
The first novel stereoselective synthesis of naturally occurring A1 (1) and A2 proanthocyanidins (2) has been achieved. The key synthetic steps involved (a) the formation of a coupled product (13 or 14) between an open chain C-ring C-4 hydroxyethoxy analogue of either (+)-catechin or (−)-epicatechin with 5,7,3′,3′-tetra-O-benzyl-(+)-catechin/-(−)-epicatechin in the presence of bentonite clay K-10, (b) removal of benzyl protecting groups under mild catalytic hydrogenation conditions to form the desired A-type compound in situ as a mixture of diastereomers via ketal/oxonium ion/carbonium ion formation, and (c) separation of the diasteromers via silica gel column chromatography. The structures of A1 and A2 proanthocyanidins were unequivocally established by analytical comparison to the natural products. Following this methodology, an additional six diastereomers of proanthocyanidins A1 and A2 have been synthesized. A plausible mechanism for the formation of the A-type linkage in proanthocyanidins has been proposed.
中文翻译:

原花青素A1,A2及其立体异构体的全合成
已经实现了天然存在的A1(1)和A2原花青素(2)的第一个新颖的立体选择性合成。关键的合成步骤涉及(a)在(+)-儿茶素或(-)-表儿茶素的开链C环C-4羟基乙氧基类似物与5,7,3之间形成偶联产物(13或14)在膨润土K-10存在下,',3'-四-O-苄基-(+)-儿茶素/-(-)-表儿茶素,(b)在温和的催化氢化条件下除去苄基保护基以形成所需的原位A型化合物通过缩酮/氧鎓离子/碳酸根离子形成非对映异构体的混合物,以及(c)通过硅胶柱色谱分离非对映异构体。通过与天然产物的分析比较,明确地确定了A1和A2原花色素的结构。按照这种方法,已经合成了另外六个原花色素A1和A2的非对映异构体。已经提出了在原花青素中形成A型连接的合理机制。
更新日期:2015-04-30
中文翻译:

原花青素A1,A2及其立体异构体的全合成
已经实现了天然存在的A1(1)和A2原花青素(2)的第一个新颖的立体选择性合成。关键的合成步骤涉及(a)在(+)-儿茶素或(-)-表儿茶素的开链C环C-4羟基乙氧基类似物与5,7,3之间形成偶联产物(13或14)在膨润土K-10存在下,',3'-四-O-苄基-(+)-儿茶素/-(-)-表儿茶素,(b)在温和的催化氢化条件下除去苄基保护基以形成所需的原位A型化合物通过缩酮/氧鎓离子/碳酸根离子形成非对映异构体的混合物,以及(c)通过硅胶柱色谱分离非对映异构体。通过与天然产物的分析比较,明确地确定了A1和A2原花色素的结构。按照这种方法,已经合成了另外六个原花色素A1和A2的非对映异构体。已经提出了在原花青素中形成A型连接的合理机制。


































 京公网安备 11010802027423号
京公网安备 11010802027423号