当前位置:
X-MOL 学术
›
Environ. Sci. Technol. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
In Situ Electrochemical Dilatometry of Phosphate Anion Electrosorption
Environmental Science & Technology Letters ( IF 8.9 ) Pub Date : 2018-11-19 , DOI: 10.1021/acs.estlett.8b00542 Daniel Moreno 1 , Yousuf Bootwala 1 , Wan-Yu Tsai 2 , Qiang Gao 2 , Fengyu Shen 3 , Nina Balke 2 , Kelsey B. Hatzell 3 , Marta C. Hatzell 1
Environmental Science & Technology Letters ( IF 8.9 ) Pub Date : 2018-11-19 , DOI: 10.1021/acs.estlett.8b00542 Daniel Moreno 1 , Yousuf Bootwala 1 , Wan-Yu Tsai 2 , Qiang Gao 2 , Fengyu Shen 3 , Nina Balke 2 , Kelsey B. Hatzell 3 , Marta C. Hatzell 1
Affiliation
|
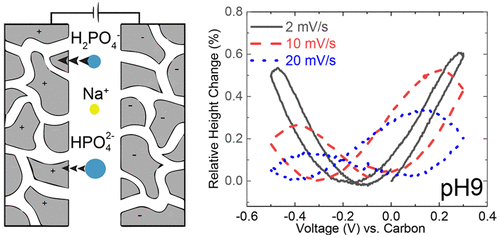
Here we investigate the competitive electrosorption of mono- and divalent phosphate anions through electrochemical desalination- and dilatometry-based experiments. Through in situ dilatometry, we monitor the strain at the electrode surface as anions and cations are electrosorbed. Strain measurements show that the presence of divalent ions promotes a greater than anticipated electrode expansion during cation (Na+) electrosorption. The expansion observed with Na+ equaled the expansion observed with HPO42–. Because the ionic radius of Na+ is smaller than that of HPO42–, the symmetric expansion suggests that divalent anions do not completely desorb during electrode regeneration, causing the adverse interactions with the cation during co-ion expulsion. This results in a decrease in desalination performance, indicated by a decreased salt adsorption capacity. Conversely, an expected asymmetric expansion during anion and cation electrosorption occurs with monovalent phosphate anions (H2PO4–), indicating that monovalent ions can be effectively replaced by the cation at the electrode surface.
中文翻译:

磷酸根阴离子电吸附的原位电化学膨胀法
在这里,我们通过基于电化学脱盐和膨胀的实验研究了单价和二价磷酸根阴离子的竞争性电吸附。通过原位膨胀法,当阴离子和阳离子被电吸附时,我们监测电极表面的应变。应变测量表明,在阳离子(Na +)电吸附过程中,二价离子的存在促进了比预期的电极扩展更大的扩展。用Na +观察到的膨胀等于用HPO 4 2–观察到的膨胀。因为Na +的离子半径小于HPO 4 2–,对称膨胀表明,二价阴离子在电极再生过程中不会完全解吸,从而导致在驱出co-ion期间与阳离子发生不利的相互作用。这导致脱盐性能下降,这表明盐吸附能力下降。相反,单价磷酸根阴离子(H 2 PO 4 –)在阴离子和阳离子电吸附过程中会发生预期的不对称膨胀,表明单价离子可以有效地被电极表面的阳离子替代。
更新日期:2018-11-20
中文翻译:

磷酸根阴离子电吸附的原位电化学膨胀法
在这里,我们通过基于电化学脱盐和膨胀的实验研究了单价和二价磷酸根阴离子的竞争性电吸附。通过原位膨胀法,当阴离子和阳离子被电吸附时,我们监测电极表面的应变。应变测量表明,在阳离子(Na +)电吸附过程中,二价离子的存在促进了比预期的电极扩展更大的扩展。用Na +观察到的膨胀等于用HPO 4 2–观察到的膨胀。因为Na +的离子半径小于HPO 4 2–,对称膨胀表明,二价阴离子在电极再生过程中不会完全解吸,从而导致在驱出co-ion期间与阳离子发生不利的相互作用。这导致脱盐性能下降,这表明盐吸附能力下降。相反,单价磷酸根阴离子(H 2 PO 4 –)在阴离子和阳离子电吸附过程中会发生预期的不对称膨胀,表明单价离子可以有效地被电极表面的阳离子替代。









































 京公网安备 11010802027423号
京公网安备 11010802027423号