当前位置:
X-MOL 学术
›
Chem. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Facile synthesis of unnatural β-germyl-α-amino amides via Pd(ii)-catalyzed primary and secondary C(sp3)–H bond germylation†
Chemical Communications ( IF 4.3 ) Pub Date : 2018-11-20 00:00:00 , DOI: 10.1039/c8cc08098d Zheng-Xin Zhou 1, 2, 3 , Wei-Hao Rao 3, 4, 5 , Ming-Hua Zeng 1, 2, 3 , Yue-Jin Liu 1, 2, 3
Chemical Communications ( IF 4.3 ) Pub Date : 2018-11-20 00:00:00 , DOI: 10.1039/c8cc08098d Zheng-Xin Zhou 1, 2, 3 , Wei-Hao Rao 3, 4, 5 , Ming-Hua Zeng 1, 2, 3 , Yue-Jin Liu 1, 2, 3
Affiliation
|
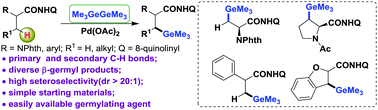
Pd(II)-Catalyzed direct C(sp3)–H germylation of α-AA derivatives with the assistance of a bidentate auxiliary for the efficient synthesis of β-germyl-α-amino amides is reported. This protocol features good generality for primary and secondary C–H bonds of aliphatic amides. Mechanistic studies show that a crucial five-membered palladacycle intermediate may play a key role in this process.
中文翻译:

通过Pd(ii)催化的初级和次级C(sp 3)–H键的芽生反应,可轻松 合成非天然β-胚芽基-α-氨基酰胺†
据报道,在二齿助剂的辅助下,Pd(II)催化的α-AA衍生物的直接C(sp 3)-H芽孢生成有效地合成了β-锗烷基-α-氨基酰胺。该协议对脂族酰胺的一级和二级C–H键具有良好的通用性。机理研究表明,关键的五元palladacycle中间体可能在此过程中起关键作用。
更新日期:2018-11-20
中文翻译:

通过Pd(ii)催化的初级和次级C(sp 3)–H键的芽生反应,可轻松 合成非天然β-胚芽基-α-氨基酰胺†
据报道,在二齿助剂的辅助下,Pd(II)催化的α-AA衍生物的直接C(sp 3)-H芽孢生成有效地合成了β-锗烷基-α-氨基酰胺。该协议对脂族酰胺的一级和二级C–H键具有良好的通用性。机理研究表明,关键的五元palladacycle中间体可能在此过程中起关键作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号