当前位置:
X-MOL 学术
›
Chem. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Palladium-catalyzed intramolecular transfer hydrogenation & cycloaddition of p-quinamine-tethered alkylidenecyclopropanes to synthesize perhydroindole scaffolds†
Chemical Communications ( IF 4.9 ) Pub Date : 2018-11-20 00:00:00 , DOI: 10.1039/c8cc09041f Bo Cao 1, 2, 3, 4, 5 , Yin Wei 6, 7, 8, 9, 10 , Min Shi 1, 2, 3, 4, 5
Chemical Communications ( IF 4.9 ) Pub Date : 2018-11-20 00:00:00 , DOI: 10.1039/c8cc09041f Bo Cao 1, 2, 3, 4, 5 , Yin Wei 6, 7, 8, 9, 10 , Min Shi 1, 2, 3, 4, 5
Affiliation
|
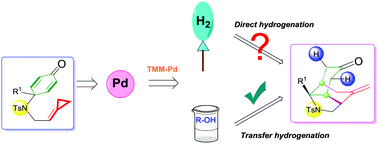
A palladium(0)-catalyzed intramolecular transfer hydrogenation and cycloaddition of p-quinamine-tethered alkylidenecyclopropanes (ACPs) to synthesize perhydroindole scaffolds has been reported in this communication. Mechanistic investigations on the basis of deuterium labeling experiments suggest that the reaction proceeded through an oxidative addition of Pd(0) into the distal bond of the ACP moiety to afford a trimethylenemethane (TMM)–Pd intermediate followed by transfer hydrogenation using alcohol.
中文翻译:

钯催化的分子内转移氢化&的环加成p -quinamine拴alkylidenecyclopropanes合成perhydroindole支架†
在该文献中已报道了钯(0)催化的分子内转移氢化和对苯二胺系链的亚烷基环丙烷(ACP)的环加成反应,以合成全氢吲哚支架。在氘标记实验的基础上进行的机理研究表明,该反应通过将Pd(0)氧化加成到ACP部分的远端键中进行,得到三亚甲基甲烷(TMM)-Pd中间体,然后使用醇进行转移氢化。
更新日期:2018-11-20
中文翻译:

钯催化的分子内转移氢化&的环加成p -quinamine拴alkylidenecyclopropanes合成perhydroindole支架†
在该文献中已报道了钯(0)催化的分子内转移氢化和对苯二胺系链的亚烷基环丙烷(ACP)的环加成反应,以合成全氢吲哚支架。在氘标记实验的基础上进行的机理研究表明,该反应通过将Pd(0)氧化加成到ACP部分的远端键中进行,得到三亚甲基甲烷(TMM)-Pd中间体,然后使用醇进行转移氢化。



























 京公网安备 11010802027423号
京公网安备 11010802027423号