当前位置:
X-MOL 学术
›
Chem. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Bioorthogonal release of sulfonamides and mutually orthogonal liberation of two drugs†
Chemical Communications ( IF 4.3 ) Pub Date : 2018-11-16 00:00:00 , DOI: 10.1039/c8cc08533a Zhuzhou Shao 1 , Wei Liu , Huimin Tao , Fang Liu , Ruxin Zeng , Pier Alexandre Champagne , Yang Cao , K N Houk , Yong Liang
Chemical Communications ( IF 4.3 ) Pub Date : 2018-11-16 00:00:00 , DOI: 10.1039/c8cc08533a Zhuzhou Shao 1 , Wei Liu , Huimin Tao , Fang Liu , Ruxin Zeng , Pier Alexandre Champagne , Yang Cao , K N Houk , Yong Liang
Affiliation
|
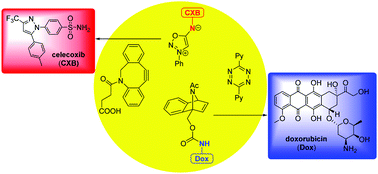
Sulfonamide derivatives have been used in pharmaceutics for decades. Here we report a new approach to release sulfonamides efficiently using a bioorthogonal reaction of sulfonyl sydnonimines and dibenzoazacyclooctyne (DIBAC). The second-order rate constant of the cycloaddition reaction can be up to 0.62 M−1 s−1, and the reactants are highly stable under physiological conditions. Most significantly, we also discovered the mutual orthogonality between the sydnonimine–DIBAC and benzonorbornadiene–tetrazine cycloaddition pairs, which can be used for selective and simultaneous liberation of sulfonamide and primary amine drugs.
中文翻译:

磺胺类药物的生物正交释放和两种药物的相互正交释放†
磺酰胺衍生物在药剂学中的应用已有数十年历史。在这里,我们报告了一种利用磺酰基苯乙烯亚胺和二苯并氮杂环辛炔(DIBAC)的生物正交反应有效释放磺酰胺的新方法。环加成反应的二级速率常数可达0.62 M -1 s -1 ,反应物在生理条件下高度稳定。最重要的是,我们还发现了 sydnonimine-DIBAC 和苯并降冰片二烯-四嗪环加成对之间的相互正交性,可用于选择性和同时释放磺酰胺和伯胺药物。
更新日期:2018-11-16
中文翻译:

磺胺类药物的生物正交释放和两种药物的相互正交释放†
磺酰胺衍生物在药剂学中的应用已有数十年历史。在这里,我们报告了一种利用磺酰基苯乙烯亚胺和二苯并氮杂环辛炔(DIBAC)的生物正交反应有效释放磺酰胺的新方法。环加成反应的二级速率常数可达0.62 M -1 s -1 ,反应物在生理条件下高度稳定。最重要的是,我们还发现了 sydnonimine-DIBAC 和苯并降冰片二烯-四嗪环加成对之间的相互正交性,可用于选择性和同时释放磺酰胺和伯胺药物。











































 京公网安备 11010802027423号
京公网安备 11010802027423号