npj Precision Oncology ( IF 7.9 ) Pub Date : 2018-11-15 , DOI: 10.1038/s41698-018-0068-8 K Okrah 1 , S Tarighat 2 , B Liu 2 , H Koeppen 3 , M C Wagle 2 , G Cheng 2 , C Sun 2 , A Dey 4 , M T Chang 4 , T Sumiyoshi 2 , Z Mounir 2 , C Cummings 2 , G Hampton 2 , L Amler 2 , J Fridlyand 1 , P S Hegde 2 , S J Turley 4 , M R Lackner 2 , S M Huang 2
|
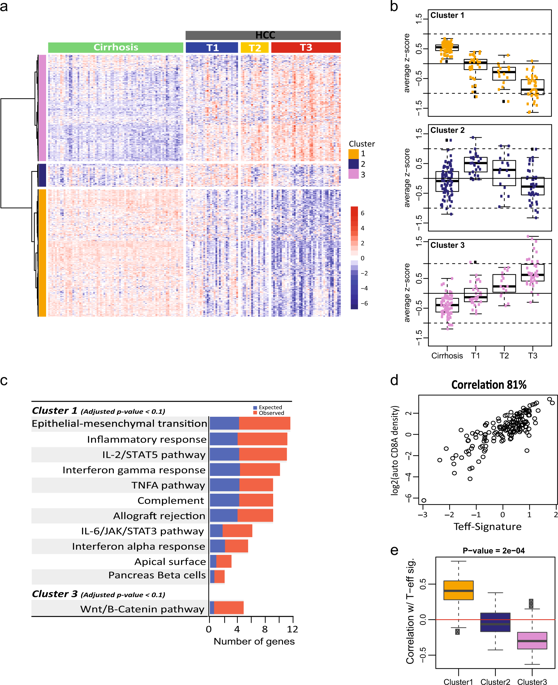
Hepatocellular carcinoma (HCC) develops in the context of chronic inflammatory liver disease and has an extremely poor prognosis. An immunosuppressive tumor microenvironment may contribute to therapeutic failure in metastatic HCC. Here, we identified unique molecular signatures pertaining to HCC disease progression and tumor immunity by analyzing genome-wide RNA-Seq data derived from HCC patient tumors and non-tumor cirrhotic tissues. Unsupervised clustering of gene expression data revealed a gradual suppression of local tumor immunity that coincided with disease progression, indicating an increasingly immunosuppressive tumor environment during HCC disease advancement. IHC examination of the spatial distribution of CD8+ T cells in tumors revealed distinct intra- and peri-tumoral subsets. Differential gene expression analysis revealed an 85-gene signature that was significantly upregulated in the peri-tumoral CD8+ T cell-excluded tumors. Notably, this signature was highly enriched with components of underlying extracellular matrix, fibrosis, and epithelial–mesenchymal transition (EMT). Further analysis condensed this signature to a core set of 23 genes that are associated with CD8+ T cell localization, and were prospectively validated in an independent cohort of HCC specimens. These findings suggest a potential association between elevated fibrosis, possibly modulated by TGF-β, PDGFR, SHH or Notch pathway, and the T cell-excluded immune phenotype. Indeed, targeting fibrosis using a TGF-β neutralizing antibody in the STAM™ model of murine HCC, we found that ameliorating the fibrotic environment could facilitate redistribution of CD8+ lymphocytes into tumors. Our results provide a strong rationale for utilizing immunotherapies in HCC earlier during treatment, potentially in combination with anti-fibrotic therapies.
中文翻译:

肝细胞癌的转录组分析揭示疾病进展和肿瘤免疫生物学的分子特征
肝细胞癌(HCC)在慢性炎症性肝病的背景下发生,预后极差。免疫抑制性肿瘤微环境可能导致转移性肝癌治疗失败。在这里,我们通过分析来自 HCC 患者肿瘤和非肿瘤肝硬化组织的全基因组 RNA-Seq 数据,确定了与 HCC 疾病进展和肿瘤免疫相关的独特分子特征。无监督的基因表达数据聚类揭示了与疾病进展相一致的局部肿瘤免疫的逐渐抑制,表明在 HCC 疾病进展过程中免疫抑制的肿瘤环境日益增强。对肿瘤中 CD8+ T 细胞空间分布的 IHC 检查揭示了不同的肿瘤内和肿瘤周围亚群。差异基因表达分析揭示了 85 个基因特征,该特征在肿瘤周围 CD8+ T 细胞排除的肿瘤中显着上调。值得注意的是,这一特征高度富含潜在细胞外基质、纤维化和上皮间质转化(EMT)的成分。进一步的分析将这一特征浓缩为与 CD8+ T 细胞定位相关的 23 个核心基因,并在独立的 HCC 样本队列中进行了前瞻性验证。这些发现表明纤维化程度升高(可能由 TGF-β、PDGFR、SHH 或 Notch 通路调节)与 T 细胞排斥的免疫表型之间存在潜在关联。事实上,在鼠 HCC 的 STAM™ 模型中使用 TGF-β 中和抗体靶向纤维化,我们发现改善纤维化环境可以促进 CD8+ 淋巴细胞重新分布到肿瘤中。我们的结果为在治疗过程中早期使用免疫疗法(可能与抗纤维化疗法相结合)治疗 HCC 提供了强有力的理由。



























 京公网安备 11010802027423号
京公网安备 11010802027423号